The concept of an analytical lifecycle has been well received in the biopharmaceutical industry. In 2016, the US Pharmacopeia (USP) advocated for lifecycle management of analytical procedures (1) and defined its three stages: method design development and understanding, qualification of the method procedure, and procedure performance verification. The US Food and Drug Administration (FDA) has published guidance on process validation with a similar division into three stages: process design, process performance qualification, and process performance verification (2). For a manufacturing…
2019
Apparent Matrix Effects in an Iduronate 2-Sulfatase Specific Activity Assay
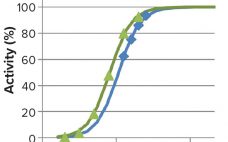
The recombinant fusion protein SHP631 consists of a chimeric monoclonal antibody binding to human insulin receptor and iduronate-2-sulfatase (I2S). This product is being developed as an enzyme replacement therapy to treat cognitive symptoms of Hunter’s syndrome. Because the current therapy (idursulfase, brand name Elaprase from Shire) cannot cross the blood–brain barrier (BBB), SHP631 is being developed to do so, enabling the presence of I2S in the brain. The enzymatic activity of this molecule is measured using the substrate 4-methyl umbelliferyl-α-L-idopyranosiduronic…
Developing an End-to-End Scale-Down Model for a Commercial-Scale Downstream Process: Enhancing Technology Transfer Efficiency
Large and complex protein molecules used as therapeutic agents are manufactured in a series of process steps that start with thawing of cell-bank vials and finish with filling and packaging (Figure 1). The cost and complexity of commercial-scale biomanufacturing processes make them prohibitive to troubleshoot or experiment at full commercial scale. Biopharmaceutical companies routinely use scale-down models (SDMs) of licensed commercial-scale processes to evaluate raw material changes, process improvements, and deviations (1) (Figure 2). Here, we outline some considerations in…
Smart Modular Package Units for Single-Use Processing: Addressing Cost, Speed, and Flexibility Challenges in Biologics Manufacturing
How to reduce costs, while still increasing speed, and flexibility in biologics manufacturing currently represent major issues for the biopharma industry. In this article, Burkhard Joksch, Product Manager Bioprocess Automation and Stuart Tindal, Product Manager FlexAct® Platform at Sartorius Stedim Biotech GmbH, detail the evolution of automation for single-use technology towards modular packaging units. They also including case studies of how these units can be used in real life cGMP processes, as well as explain the manufacturing and industry benefits…
Immunotherapy for Cancer: Why Fight Only Half the Battle?
Immunotherapy has made incredible strides over the past several years. Studies have shown it to be effective in treating many diseases that attack the immune system, such as multiple sclerosis, rheumatoid arthritis and psoriasis, and (most recently) cancer. With the latter in particular, although immunotherapy is proving to be effective for some patients, most do not respond to the treatment. It is time for the industry to reevaluate how it approaches immunotherapy especially for treatment-resistant patients. In the fight against…
BioProcess International West: Event Planner
BioProcess International US West is the leading phase-based bioprocessing event for accelerating promising biologics toward commercial success. Whereas most bioprocessing event sessions are broken out by departmental function — e.g., analytical, upstream, and downstream — the BPI US West sessions are defined by stage of development, providing drug developers a unique opportunity to break down silos across multiple departments to discuss today’s leading solutions toward improving the speed, lowering the cost, and increasing the quality/safety of biologics in development. Each…
Continuous Chromatography: Experts Weigh in on the Possibilities and the Reality
Discussions of continuous processing in the biopharmaceutical industry are an important part of current efforts toward intensifying bioproduction and bioprocessing. Biomanufacturers are looking at all components of their development and manufacturing processes for ways to reduce the size of their facilities, lower costs, and increase speed and flexibility of operations. Increasing options for and availability of single-use technologies have been major enablers of myriad attempts to improve efficiencies. Although the general consensus may still be that single-use components are more…
Manufacturing Insights: Interviews from BWB 2018 Highlight Perspectives on Streamlining Manufacturing Timelines
Addressing manufacturing and technologies strategies to accelerate market entry is one of BPI’s highlighted themes for 2019. In partnership with our conference colleagues in Informa’s KNect365 division, this already has been a shared theme, reflecting the general goals of the industry and related advice from its regulators. BPI’s summer 2018 preconference ebook included interviews by Dan Stanton (editor, BioProcess Insider) with speakers previewing their talks for the BPI Conference in Boston on 7 September 2018. Two of those conversations focused…
Aspects of Acceleration: Biomanufacturers Need Smart Strategies to Speed Products to Market
No matter what the industry, it’s widely accepted that slow-moving companies give their nimbler competitors an advantage, allowing them room to dominate the market even if their products are not superior. “Me-too” products and their sponsors often are seen as followers rather than leaders — even if they offer improvements over what is already available. Fast movers are flexible and adaptive to a dynamic business environment. They capitalize on opportunities and navigate risks and challenges by responding quickly to changes…
January-February From the Editor
Greetings from the BPI editorial staff — we wish you a healthy and prosperous 2019! Beginning with a combined January–February issue somehow makes the year seem to be speeding by right from the start. We are immersed now in the first radical redesign of our magazine since the publication’s founding in 2003. Some aspects proceed smoothly; others, not so much. You see with this issue a new cover design that ties us in with our KNect365 event brands. You’ll see…