It’s hard to believe that just six years ago, BioProcess International published its first cell therapy supplement, which included just one article on “cell therapy bioprocessing” (1). At the time, most such processing was conducted in special clinical laboratories and academic institutions. As BPI continued to cover this relatively new segment of the biopharmaceutical industry, we heard more about “the product is the process” and “scale out instead of scaling up.” After many trials, errors, and milestones, regenerative medicine has…
February 2017 Featured Report
Growth Projections for the Regenerative Medicine Market
One of the fastest growing medical markets with great potential, regenerative medicine treats chronic diseases that were once untreatable. Therapy using live cells is increasingly used to replace damaged tissue, deliver gene therapies to target tissues and organs, and stimulate self-healing along with a number of other applications. View the full article below – Login Required Reference 1 Regenerative Medicines Market: Global Opportunity Analysis and Industry Forecast, 2015–2022. Allied Market Research: Pune,Maharashtra, India: August 2016; www.alliedmarketresearch.com/regenerative-medicines-market. 2 Chartrain NA, Williams…
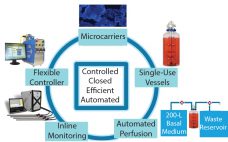
Expansion Platform Components
I first met Chris Mach at the Biotech Week Boston conference in October. We discussed the challenges that biomanufacturers are facing in cell expansion, especially in three specific areas in scale-up systems. View the full article below – Login Required
Platform Solutions for Cell Therapy Manufacturing
Advances in cell therapy have resulted in significant progress toward treating some widespread and difficult diseases, many of which represent unmet medical needs. For example, phase 3 clinical trials are already under way for therapies based on mesenchymal stem cells (MSCs), including therapies for graft-versus-host disease, acute myocardial ischemia, and chronic obstructive pulmonary disease (COPD) (1–3). Successful cell therapy treatments for such afflictions will be not only significant medical breakthroughs, but also in very high demand. However, their commercialization is…
Scalable Purification of Viral Vectors for Gene Therapy: An Appraisal of Downstream Processing Approaches
Gene therapy is the transfer of genetic material to a patient’s cells to achieve a therapeutic effect. Therapeutic DNA is largely delivered using viral vector systems based on adenoviruses (Ad), adenoassociated viruses (AAV), and lentiviruses (LV). With the application of such viral vectors as clinical therapeutics growing, scalable commercial processes (particularly for purification) are being investigated and optimized to best ensure that critical quality attributes (CQAs) are retained. Herein we review viral vector purification techniques and the effect of different…
3D Bioprinting Possibilities and Challenges
Three-dimensional (3D) bioprinting is the newest addition to the regenerative medicine family. Now within the industry dedicated to providing more personalized drug products, this new additive-manufacturing technology has the potential to truly focus on individual tissue repair and replacement. In a short period of time, 3D bioprinting has been applied in studies using bones, blood vessels, composite tissues, vascular grafts, tracheal splints, cartilaginous structures, heart tissue (e.g., two-valve heart), and vaginal organs (1). View the full article below – Login…