The BPI West conference is always a “candy store” of timely presentations and key networking opportunities for an editor who cannot be in all places at once. This year’s event in San Francisco (27 February – 2 March) was no exception. Nearly 900 delegates were joined by 86 exhibitors and 48 poster presentations. I’m inviting a number of speakers to convert their talks into manuscripts for BPI. In focusing on late-stage processes and commercial trends at this year’s event, I…
April 2017
April Spotlight
WHO’s Most-Wanted List: Deadliest Bacteria In February, the World Health Organization (WHO) published its first-ever list of “priority pathogens,” a catalogue of 12 families of bacteria that pose the greatest threats to human health. In particular this list highlights the threat of gram-negative bacteria that are resistant to multiple antibiotics. It is intended to guide and promote research and development (R&D) of new antibiotics. “This is a new tool to ensure R&D responds to urgent public health needs,” said Marie-Paule…
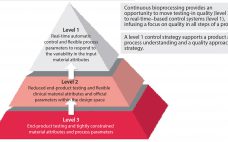
The Industry’s Hesitation to Adopt Continuous Bioprocessing: Recommendations for Deciding What, Where, and When to Implement
The US Food and Drug Administration has stated its appreciation of continuous bioprocessing (CBP), and some studies have shown that it can save manufacturers time and money. However, the bioprocessing industry is still reluctant to implement continuous bioprocessing right away. It will be interesting to see which companies will be among the first-movers to harness the competitive benefits. Although few biologics today are made using CBP-enabled equipment (e.g., advanced bioreactors), the industry is changing. For biologics already in production, it…
BioPhorum Operations Group Technology Roadmapping, Part 3: Enabling Technologies and Capabilities
Although great strides have been made over the past 20 years to increase the productivity and robustness of manufacturing processes for biopharmaceuticals, the cost and complexity of their development and manufacturing remain high, especially in comparison with those of small-molecule pharmaceuticals. Process improvements are required to increase patient access while maintaining the viability of an R&D-driven biopharmaceutical industry. Facility productivity, cost of goods (CoG), and capital investment all have significant margins for improvement. Such goals can be achieved not only…
Aggregation from Shear Stress and Surface Interaction: Molecule-Specific or Universal Phenomenon?
Exposure to solid–liquid and air–water interfaces during production, freezing and thawing, shipment and storage of protein therapeutics may be a contributing factor in their degradation (e.g., aggregation, fragmentation) (1, 2). Surface exposure, particularly during manufacturing processes, often is accompanied by various degrees and durations of shear stresses originating from fluid flow and acting on proteins at interfaces. The magnitude and duration of shear rates depends on velocity gradients within each solution and varies significantly among manufacturing steps. On the low…
Strategies for Successful Sample Transfer
Nadine Ritter is president and senior analytical advisor of Global Biotech Experts, LLC and a long-time member of BioProcess International’s editorial advisory board. At a recent CASSS North American CMC Strategy Forum called “Methods on the Move: Addressing Method Transfer Challenges,” she discussed the biopharmaceutical industry’s logistical challenges of analytical test samples for drug substances and products. At the conference, BPI’s editor in chief Anne Montgomery met with her to discuss some key points of this topic. Logistics Challenges Montgomery:…
How to Set Up a Perfusion Process for Higher Productivity and Quality
Biotherapeutic proteins usually are produced by either fed-batch or perfusion processes. Perfusion manufacturing can provide much higher levels of productivity than fed-batch systems can, thereby reducing production costs. A 2013 study showed that perfusion is more cost effective than fed-batch processes for most combinations of titers and production volumes (1). Moreover, because a perfusion process is much closer to steady state than is a fed-batch process, it often produces a more consistent product — especially for molecules that are sensitive…
Enabling Faster Workflows with Protein Purification Technologies: Improvements in Chromatography and Electrophoresis
Purification of recombinant proteins is a critical step during protein therapeutics development. Protein therapeutics have a number of classifications based on their potential applications, including use as vaccines and diagnostics as well as for enzymatic, regulatory, or targeting activities (1). For all such applications, identifying and verifying protein purity is most important. Whether proteins themselves are therapeutics or the target proteins of interest, effective purification is essential in drug development. Since the introduction of recombinant proteins in the early 1980s,…
Going After a Moving Target: New Production Methods Aid in the Flu Fight
The traditional method of manufacturing vaccines for influenza involves infecting hens’ eggs with the virus, then harvesting and purifying the large amounts of virus that they produce as a result. It’s time-consuming and expensive, requiring large specialized facilities for production. With the advent of genetic engineering and decades of improvement in protein production through cell-line engineering and industrial culture, it was only a matter of time before the vaccine industry saw the real value in modern biomanufacturing instead (1, 2).…