Some issues of BPI more than others emphasize to me the interdisciplinary nature of our audience. One example: I am happy to bring you the latest consensus paper from the CMC Forum series. In its discussion of host-cell protein control strategies, the authors write of their goal to “bring industry, academics, and regulators together for generating integrated perspectives on HCPs and their control.” Those three groups — industry, academics, and regulators (government) — constitute three major categories of our readership…
September 2016
September Spotlight
Women In Bio Launches Executive-Level Certification Program To prepare women in life sciences for roles on boards of directors, the national Women in Bio (WIB) organization has launched a program for executive-level board certification. “Women are significantly underrepresented on the boards of life science companies,” says Kristi Sarno, national president of the group, “and the Boardroom Ready program will ensure that more women are qualified, willing, and proactively seeking board memberships. This new initiative is an important step toward promoting…
Special Report: GE Bioprocess Insights
Introduction Accelerate activity. Improve predictability. Drive higher process efficiency. Increase quality. Lower cost of goods sold (CoGS). Secure supply. In an era where the biomanufacturing wheels turn faster by the day, where the stakes are higher and the choices seemingly endless, it is easy to become overwhelmed. How can you make good biomanufacturing decisions and develop robust long-term strategies when the environment is constantly changing? Whether a political shift affects your product or market, a natural disaster disrupts your supply…
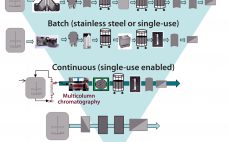
Standardized Economic Cost Modeling for Next-Generation MAb Production
Historically, in generating material for clinical testing during antibody process development, emphasis was placed on efficacy, product quality, regulatory compliance, and speed. As the biopharmaceutical industry has matured (and with increasing competition), emphasis has shifted toward cost optimization and manufacturability. Reducing the costs of medicines for patients and payers (thereby broadening access to drugs) is now a key driver during development of new therapies as well as modernizing processes for existing molecules. Cost reduction includes providing robust manufacturing processes that…
Quality By Design for Monoclonal Antibodies, Part 2: Process Design Space and Control Strategies
Process design space and control strategy are two fundamental elements of quality by design (QbD) that must be established as part of biopharmaceutical development and regulatory filings. Like all of QbD, they are interconnected and iterative. Both are based on knowledge gained during product and process development — but both need to be in place (in a potentially very limited form) when a company begins to manufacture drug substance for clinical trials. Part 1 of this discussion appears on pages…
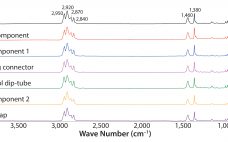
Investigation of Foreign-Particle Contamination: Practical Application of FT-IR, Raman, and SEM-EDS Technologies
The presence of visible foreign particulate matter is considered a critical defect in parenteral products and one of the main reasons they can be recalled (1). Foreign particles present during any stage of manufacturing are considered to be contaminants and can impose a risk to the control of the manufacturing processes (2). For those reasons, particle contamination arising in any manufacturing step initiates a nonconformance or out-of-specification observation. That requires an investigation to identify root cause so as to mitigate…
Science, Risks, and Regulations: Current Perspectives on Host Cell Protein Analysis and Control
State-of-the-art analytics guide process development by providing companies with thorough understanding, effective removal, suitable control, and comparability assessment after process changes of host cell proteins (HCPs) in recombinant biotechnology products. An array of analytical techniques and approaches can be used to establish control strategies for host cell proteins. Techniques used for HCP characterization and comparability include two-dimensional (2D) gel electrophoresis with a range of stains, 2D immunoblotting, 2D high-performance liquid chromatography (HPLC), 2D difference gel electrophoresis (DIGE), and increasingly mass…
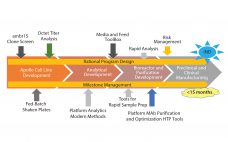
Rapid Development of High-Quality, Robust Mammalian Cell Culture Manufacturing Processes
With increasing industry emphasis on providing both rapid and robust processes, companies are reaping the benefits of new tools for risk management and process analytical controls. As a current example of these approaches, Fujifilm Diosynth scientists have accelerated the development process from gene to finish by shortening the timeline, incorporating quality by design (QbD) principles, and designing the process to be as robust as possible. When the Apollo mammalian expression cell line was introduced three years ago, the time from…
Performance Qualification of a Single-Use, Stirred-Tank Bioreactor with CHO-S Cell Culture
The increasing role and importance of cell culture in biophamaceutical manufacturing has led to considerable research and development (R&D) into bioreactor design and performance in recent years. As a result, a greater understanding of bioreactor fluid dynamics and critical physical parameters is now essential to maximize cell growth and productivity. Stirred-tank bioreactors are especially important in this development process because of their favorable properties in areas such as mixing efficiency and homogeneity, energy transfer, and scalability. The design and manufacture…
Harnessing the Power of Big Data to Improve Drug R&D
Like many other industries, biotechnology is being transformed by the emergence of big data — extremely large data sets that can be analyzed to reveal patterns, trends, and associations — and advanced analytics. Information from multiple sources such as electronic health records, payer claims, and mobile health platforms is growing exponentially. When used and harnessed properly, these data can boost the efficiency of drug research and development (R&D) in three critical areas: early R&D investment, drug development, and personalized medicine.…