Just a few years ago, my requests for manuscripts detailing logistical considerations for cell-therapy manufacturing were met with puzzlement. The assumption seemed to be that such processes already existed for commercial biopharmaceuticals and would simply be adapted later. In quite a few cases, adopting a commercial mind-set did not appear to be a goal at all, with hands-on practitioners used to applying procedures in hospital settings. For those of us who remember early discussions about commercialization and cost-containment for protein…
October 2015 Supplement
Cell Therapy Scaling: Beyond the Biology
Few other areas of medical research have been the source of as much promise (and hype) as cell therapies. Therapies that use engineered or repurposed versions of our own cells have inspired researchers and media alike. However, the pace at which effective therapies have made it out of laboratories and into clinical practice has not met the world’s high expectations. Although the biology has continued to press onward, the gap between R&D and commercialization remains substantial. In 1957, the first…
Manufacturing Human Induced-Pluripotent Stem Cells for Clinical Application
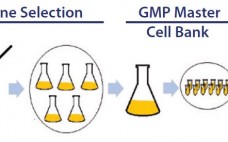
The reprogramming of human somatic cells into induced pluripotent stem cells (iPSCs) offers tremendous potential for cell therapy, basic research, disease modeling, and drug development. Human iPSCs can be generated in culture, expanded, and then used to manufacture clinical-grade cells of almost any adult cell type. Given their great capacity for self-renewal, they are attractive as input materials for current good manufacturing practice (CGMP) operations. For human iPSCs to fulfill their therapeutic potential, however, it is necessary to develop a…
Cost-Effective Process Development for Plasmid DNA Manufacture: Evaluation of Single-Use Technologies to Support Escherichia coli Culture
DNA-based gene therapy products have been in clinical development since the 1990s. But over the past 24 months, the overall demand and therapeutic applications for plasmid DNA (pDNA) have rapidly grown and expanded. Currently, pDNA can be used directly as a therapeutic agent (e.g., in gene therapy or generation of vaccine antigens) and indirectly for a range of applications. Those include its use as a critical starting material for transient transfection to produce both viral-vector constructs (e.g., lentivirus or adenoassociated…
Planning for Commercial Scale of Cell Therapy and Regenerative Medicine Products, Part 2: Clinical Efficacy, Reimbursement, and Needle-to-Needle Logistics
Cell therapy is an emerging pillar in healthcare with the potential to provide curative solutions to a wide range of indications. The biological complexities through which cell technologies exert their clinical impact (especially those used in immunotherapies for cancer) provide opportunities for novel modes of immune regulation, cell targeting, and payload delivery. Cells also can serve as vehicles for genetic content, which the gene therapy industry is now investigating. Since early 2004, Invetech has worked with organizations dedicated to cell…
Mapping Success for Commercial Cell Therapy Manufacturing
Commercializing cell therapies can be much more challenging than commercializing traditional pharmaceuticals and biologics. Cell-based drug products are significantly more complex than protein or small-molecule drug products. Their mechanisms of action and product attributes are also more complex. Cell therapy product attributes rely heavily on the associated manufacturing processes. Process changes can influence products in ways that may not be discernible until their effects on efficacy effects become evident. Product characterization is critical, but cell therapy products are living organisms…
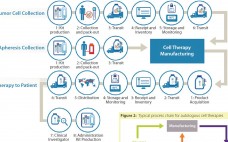
The Cell Therapy Supply Chain: Logistical Considerations for Autologous Immunotherapies
Among the basics of building a successful logistics strategy for the management of cell-based material, some better-known and important factors to consider include selecting the right dry-shipping unit, qualifying that container for a particular payload and shipping configuration, choosing an appropriate data logger, creating a chain of custody, evaluating a transit carrier, and anticipating potential problems inherent in shipping at cryogenic temperatures (1). Here, I’d like to go beyond those basics to address some lesser-known considerations. These factors may be…