Among the basics of building a successful logistics strategy for the management of cell-based material, some better-known and important factors to consider include selecting the right dry-shipping unit, qualifying that container for a particular payload and shipping configuration, choosing an appropriate data logger, creating a chain of custody, evaluating a transit carrier, and anticipating potential problems inherent in shipping at cryogenic temperatures (1). Here, I’d like to go beyond those basics to address some lesser-known considerations. These factors may be hidden in the background, but they can play a major role in the success or failure of a clinical trial as well as the long-term efficacy of a cell-based commercial product. They include standardization, package and shipping qualification, equipment validation, process qualification, and documenting the chain of custody.
Several unique logistical challenges arise for autologous cell therapies — those that use a patient’s own cells for the manufacture of a treatment that is then administered to that patient only. By contrast, allogeneic cell therapies are derived from unrelated donor(s) and administered to a relevant population of patients. All of these considerations apply to both types of cellular therapies.
The Unique Complexity of Autologous Therapy
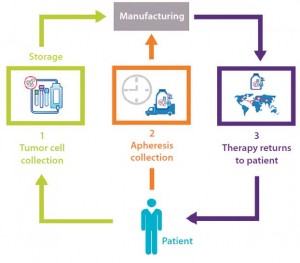
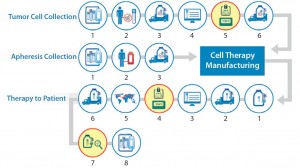
Figure 1 maps out the multiple paths traveled by different factors that must come together seamlessly for an autologous cell therapy product to be successful. From collection of base cells through production and back to the waiting patient, this process has become increasingly complex. That is not because regulatory or other demands have intensified, but rather that as we have gained more experience, we have discovered additional challenges that must be overcome to ensure success. Throughout this discussion, I will refer back to the flow chart in Figure 1 to illustrate where in the chain of custody these challenges occur and need to be addressed.
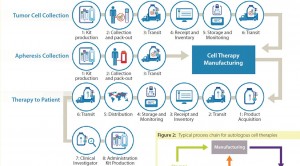
Figure 2 represents a fairly typical process chain for a cancer-related autologous cell therapy. Such therapies require two separate cell collections from a patient: a tumor and a dendritic cell (apheresis). Thus, a process for receipt, storage, and distribution of the finished product is vital to success. To define that supply chain in greater depth, Figure 2 details the three high- level processes from the chart.
Tumor Cell Collection: From a logistics perspective, the entire autologous cell therapy drug-production process begins with creation of a kit for collecting tumor cells and securely identifying the resulting sample with a specific patient. A unique identification number is assigned that will be used throughout the entire process of collection, manufacturing, distribution, and administration. Such procedures ensure that the right product is infused into the right patient.
This tumor-collection kit will include a qualified shipper for transporting tissue to an interim storage point. Using this kit, clinical staff will collect a tumor sample and package it for transport (by a common carrier) to a storage facility, where it will be received, inventoried, and stored until ordered for manufacturing. At that point, it will again be packaged in a qualified shipping unit and transported to the point of manufacture, where it is again stored until manufacturing begins.
Apheresis Collection: Timing and logistics are critical with regard to apheresis collection because an autologous cell therapy manufacturing process begins in earnest upon receipt of the resulting dendritic cells. Unlike the tumor-collection process, no interim storage step interrupts the progress of this sample. As with the tumor collection, however, the process begins with a kit that includes all components required for collection.
This kit includes patient-specific identification labels and collection containers as well as a qualified shipping container. Dendritic cells are shipped by a common carrier with the addition of an important step: notification of the manufacturer that the shipment is on the way. The resulting shipment is received by the manufacturer, which confirms the patient identity and begins the manufacturing process.
Therapy Returns to Patient: Once manufactured, autologous cell therapy doses are cryopreserved and loaded into a qualified dry-shipping unit for transport by a common carrier to a distribution facility, where the material is received and inventoried. When that therapy is packaged to leave the manufacturing site, it is “acquired” for distribution and begins the journey of a drug to a patient. At the distribution center, individual doses are inventoried and stored in a vapor-phase nitrogen freezer until requested by a clinical investigator or physician for patient use. Each requested dose is shipped in a qualified dry-shipping unit by common carrier to the clinical site for administration.
Standardization
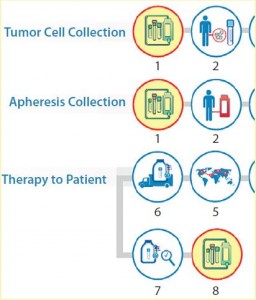
It is important to standardize as many processes and procedures as possible in the development of a logistics strategy. Some processes will remain outside your direct control. For cell collection, drug preparation/processing, and product administration, kits can be a very effective tool by which a company can define materials needed and drive consistency in the execution of these processes. Figure 3 highlights those steps in the autologous cell process (detail from Figure 1) where an opportunity exists to use kits such as those being assembled in Photo 2 to manage standardization.
The value of such kits as standardization tools increases as a cell therapy candidate traverses the advanced clinical trial phases. Early stage (phase 1 and 2a) trials often involve only one or two clinical sites. That limited number of sites allows for close coordination with a product developer to ensure that materials handling is coordinated and consistent. But a challenge arises as such products move through phase 2b and 3 trials. Those studies often include 20–50 clinical trial sites, so it becomes far more difficult to ensure that each one follows the exact same procedures for cell collection, product preparation, and administration. One key means of standardizing those processes is to have the clinics use specially designed kits for cell harvesting (collection kits) and for delivering finished drug doses to patients (administration kits).
The use of a kit ensures that the same materials will be used in each process, that instructions are available for each procedure, that labeling is consistent, and (in the case of collection kits) that a qualified shipping solution is used for transporting critical patient cells following their collection. The degree to which premade kits affect the outcome of a clinical trial depends on the complexity of the process they address. Providing an administration kit for a product that requires surgical implantation is far more critical than for one that is introduced through intravenous administration. In every case, however, the goal is to reduce process variation and ensure that clinical results reflect the efficacy of a given therapy rather than variations in its handling.
Package and Shipping Qualification
Successful transit of material is a primary objective of any logistics strategy. Consider that the simple flow diagram in Figure 1 contains five different shipments of three different materials at four different temperatures:
- A patient’s tumor (or other applicable tissue) is collected and shipped at controlled ambient temperature to a storage facility for preservation at –80 °C.
- When needed, that patient tissue is then shipped on dry ice to the cell therapy manufacturing facility.
- The same patient’s apheresis collection is shipped under refrigeration (2–8 °C) directly to the manufacturing facility.
- Finished therapeutic doses are then shipped in bulk from the manufacturing facility to a storage/distribution point using dry-shipping containers at cryogenic temperatures.
- Doses are then shipped (again in dry-shipping units) a final time to the clinical site for patient administration.
For each of those five transit points, it is critical to use qualified shipping solutions that are designed to meet the specific requirements of each individual payload for temperature, duration, and environmental and handling extremes that may be encountered during transit. Those specifications are not to be confused with the blanket qualifications often issued by the manufacturers of shipping solutions. Such information can be useful in guiding your choice of possible solutions for testing. But they fall short of the rigorous testing needed to ensure that material being shipped will reach its destination in perfect condition.
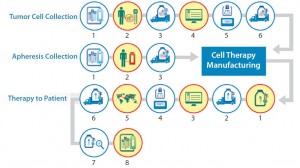
Figure 4: Logistics complexity of an autologous cell therapy (detail)— shipping and packaging qualification
A qualified shipping solution is critical to ensure that your cell therapy is judged on its clinical efficacy rather than based on deficiencies created by packaging or shipping failures. Figure 4 highlights the process points (detail from Figure 1) at which shipping and packaging qualification are needed.
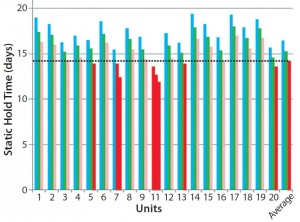
Figure 5: Static hold time for 20 dry-shipper units (all brand new, same model, and same lot) — dotted line delineates the minimum acceptable static hold time of 14 days; cyan = initial qualification (average 16.46 days); green = qualification with logger (average 15.27 days); pink = qualification with logger and payload (average 14.17 days); red = results lower than minimum acceptable
It is imperative that each individual dry-shipping container be tested because the units can vary significantly. This is not the case only for different makes and models, but also for gauging performance of shippers from the same model and lot. Figure 5 displays the static hold times for 20 dry-shipping units, all of which were brand new and the same model, from the same manufacturing lot. When those prequalified new shipping units were tested after installation of a data logger and addition of a payload, five of them (in red) failed to meet the minimum hold time indicated by the dotted line on the graph. That illustrates the variance in new shipping units right out of the box. Note that each element of the shipping configuration (data logger, rack, baffles, packaging, and product container) will influence hold time. To obtain a true understanding of the expected performance of a given dry-shipping unit, it must be validated with all those elements in place.
Beyond the shipping unit itself, three key variables influence the amount of temperature hold time required for each shipment. The first is the time it takes for an international shipment to clear customs. In most cases, customs can be cleared in 24–48 hours, but in some countries that can take considerably longer. Clearance times vary not only by country, but also with the volume being processed on a given day, delays caused by documentation problems, local holidays, weekends, and inexperience on the part of a given agent, just to mention a few possibilities. Companies must calculate a safety margin into their products’ temperature-hold requirements.
Second, dry-shipping units often are used for temporary storage at the sites of patient administration. How much time will be needed to prepare and administer a given therapy to the patient once it has arrived at the clinical site? Finally, you need to consider the amount of temperature hold that can be lost in mishandling. Dry-shipping units are designed to be used in an upright orientation. Any deviation from vertical will rob the unit of valuable hold time. For example, a unit that is tipped over on its side for eight hours can lose as much as half of its hold-time capability. Given these variables, it is important that you choose dry-shipping units that allow for the longest hold times reasonable and that you validate them under both static and dynamic conditions (Figure 5).
Although shipping of cryopreserved materials may be the most extreme logistical challenge, it is equally important to ensure that all biologics are shipped in qualified containers designed to meet the material’s temperature and duration requirements. The key to success is finding a solution that meets temperature and duration requirements in a procedure that can be easily implemented by the individuals who will perform the pack-out procedure. In late-stage trials, that may happen in hundreds of locations involving people with limited  experience in the transport of such materials. So the best solutions are those that are least complicated; the more complex a process, the more likely it is to have deviations and failures. Unfortunately, the easiest solutions are often the most costly, so you will need to evaluate cost against the risk of loss for each shipping requirement.
It is also important to consider where and when you are shipping your materials. A qualified solution that works in Maine in January may not work in Arizona in August. Among several available options, the two most common include creating summer and winter shipping profiles and creating a universal shipping solution. Establishing summer and winter profiles generally is the most cost-effective approach, but doing so can require tricky decision-making for changing seasons and weather patterns. A universal solution can be more costly, but it will be considerably easier to manage.
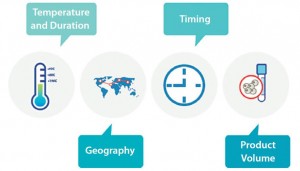
Figure 6: Considerations for shipping autologous cell therapy products include temperature and duration requirements, when and where the products are shipping, and the volume of drug being shipped.
Volume is the final consideration. Autologous cell therapies are unique in that human cells are their primary active pharmaceutical ingredient (API) and must be harvested, shipped, and incorporated into the drug- manufacturing process within a very tight window of time. That is generally a manageable process during clinical testing, but it becomes a monumental logistical challenge for commercial operations. In autologous production, the manufacturing process begins when cells are harvested. So you will need an effective scheduling and tracking system and a dependable way to transport viable materials. There is no way to manage production without tight scheduling and tracking processes in place. It is wise to begin laying ground for both during phase 2 studies, and it will be imperative for you to test commercial-volume solutions by phase 3.
Storage Equipment Validation
Validation of equipment used to maintain such valuable materials is an absolute requirement at every stage. Just as the shipping units can vary in performance and must be qualified, so too can storage equipment. It also must be validated to perform in the specific way that it will be used relative to the materials involved. Figure 7 highlights the need for equipment validation in the process- flow diagram.
For example, consider a finished drug product with a maximum storage temperature of –150 °C that will be stored in a vapor-phase dewar (Photo 3). Temperature variations occur in all vapor-phase vessels, and their interior temperature is always colder at the bottom and warmer at the top. To determine the optimum storage location inside such a tank, product developers must validate the vessel with multiple probes to determine its interior temperature gradient.
Only a portion of a given vessel — e.g., the area at or below the –150 °C level — will be appropriate for finished-product storage. A custom probe will have to be placed in that location for appropriate material monitoring. Specific validations should be performed on all equipment used for storage and handling of the finished drug product and also any of its constituents: freezers, refrigerators, liquid-nitrogen freezer vessels, and utility carts.
Process Qualification
In much the same way that we validate equipment to ensure that it will meet requirements, we also must test the procedures used for materials handling. For any point at which temperature-sensitive material is physically handled, a process qualification should be considered. Figure 8 shows areas of the process flow where this needs to be evaluated.
In general, process qualification ensures that the integrity of material is maintained while it is outside of qualified/validated equipment and being handled by people. Process qualification ensures that such materials are maintained within acceptable temperature ranges as well as within time-out-of-temperature standards. Process qualifications are always a good idea, but they are strongly recommended when material volumes are small (<2 mL), temperature windows are particularly tight (e.g., no warmer than –150 °C, no colder than –180 °C), and/or significant handling is involved (e.g., repackaging or labeling in large batches).
It is also important to consider the cumulative effects of temperature variations on your product. For example, with a product stored in cryovials, assume that those vials are stored 50 units to a box and stacked four boxes to a rack. Each time a dose is removed from storage, that rack is moved into an ambient environment. All vials in the rack — not just the one removed — are briefly exposed to a (short-duration) temperature excursion (Photo 4).
Whereas a great deal of effort is often expended in determining the effect of a single temperature excursion, far less attention is paid to the effects of cumulative exposure. In the example above, the first dose is exposed only once, but the last dose may be exposed 200 times. Although the actual handling times vary by product, it is good practice to develop processes that minimize product exposure and then to validate those processes to ensure that they do indeed meet your specifications.
Chain-of-Custody Documentation
With all cell therapy drug products, a chain of custody begins at the point of manufacture and concludes with administration to a patient. Autologous drugs require that chain to extend backward to collection of the constituent cells used for drug production. In all cases, chain-of-custody documentation must capture the location, security, and temperature of materials at all times. That chain of custody encompasses every process step in Figure 1.
Given that a chain is as strong as only its weakest link, documenting the chain of custody necessitates that all processes be given equal weight with regard to collecting and documenting accurate and complete data. A loss of documentation at any point renders the entire chain inadequate.
Maintaining suitable documentation of a custody chain can be far more difficult than it would seem. Because processes occur in different settings and are performed by multiple individuals at different organizations, a robust procedure must be in place to ensure that the requisite data are compiled and collected in a standardized way. The resulting documentation must be maintained at a per-dose level and be readily available for review whenever necessary.
And special consideration must be given to the last link in this chain: the point of patient administration. The clinical site — where the final leg of storage, processing, and administration occurs — is invariably the most difficult area for consistent collection of necessary documentation.
Plan Ahead for Success
All these logistical challenges should give you pause. Every company wants its cell therapy to be successful. By informing you well in advance about issues you are likely to face in delivering a cell therapy to patients, I hope to help you prevent unnecessary delays and costs as you progress. An experienced logistical partner can help smooth the entire round trip of an autologous cell therapy: from initial collection of cells to delivery of a finished dose back to the waiting patient who needs it.
Reference
1 O’Donnell D. Commercially Successful Cell Therapies: Navigating The Ultra Cold Chain Distribution Minefield. Fisher BioServices Blog 17 June 2013;
http://blog.fisherbioservices.com/bid/304873/Commercially-Successful-Cell-Therapies-Navigating-the-Ultra-Cold-Chain-Distribution-Minefield
Further Reading
O’Donnell D. Cell Therapy Logistics: Beyond the Basics. Fisher BioServices Blog 30 April 2015; http://blog.fisherbioservices.com/cell-therapy-logistics-beyond-the-basics
Bezawada-Joseph P. Cold Chain Qualification: 5 Questions You Must Ask When Shipping Biologics. Fisher BioServices Blog 14 August 2013;
http://blog.fisherbioservices.com/bid/326748/Cold-Chain-Qualification-5-Questions-You-Must-Ask-When-Shipping-Biologics
Dan O’Donnell is area director of cell therapy logistics for Fisher BioServices (a Thermo Fisher Scientific brand), 14665 Rothgeb Drive, Rockville, MD 20850; 1-301- 204-9431; dan.h.odonnell@thermofisher. com; www.fisherbioservices.com.