Introduction by William P. Flanagan Biopharmaceutical development and manufacturing demand scalable processes that can be quickly developed, easily implemented, and smoothly transferred to production. Disposable, ready-to-use technologies play a crucial role in providing flexibility to support agile biomanufacturing operations. Single-use systems provide process efficiencies by removing steps such as cleaning and cleaning validation, thus allowing for faster change-over between manufacturing runs. The biopharmaceutical industry is increasingly adopting single-use approaches, and the global market for such bioprocessing tools is expected to…
December 2015
Spotlight
New Sandoz Biosimilar Drug-Product Facility In September 2015, Sandoz (the generics and biosimilars business of Swiss drug maker Novartis) opened its BioInject biomanufacturing facility in Schaftenau, Austria. Over €150 million of investment is creating 100 highly skilled jobs in a state-of-the-art facility for manufacturing prefilled syringes and devices for both biosimilars and novel biologics. Staff can fill 18,000 syringes/hour and package 100 syringes/minute. In welcoming guests at the opening, Carol Lynch (global head of biopharmaceuticals and oncology injectables at Sandoz)…
From the Editor
The BioProcess International Conference took place during the last week in October in Boston, MA — when technical editor Cheryl Scott and I enjoyed meeting with so many of our readers, authors, and advisors as well as the BPI sales staff. We always receive good suggestions from our editorial advisors in our annual meeting with them, and the technical presentations help us craft the final version of our 2016 editorial calendar. As always, congratulations to our Informa colleagues at IBC…
Bioindustry Researchers Take Strides in Preventing Diseases: Developments in Genetic Diagnosis and Therapy
Johnson & Johnson’s pharmaceutical research arm recently launched a drug-development project that will intercept diseases before patients even realize they’re sick. In the company’s words: “The future of healthcare will increasingly depend on identifying and correctly interpreting the earliest signals of disease susceptibility, preventing or intercepting disease before it even begins.” (1). The first targets include Alzheimer’s, some forms of cancer, heart disease, rheumatoid arthritis, multiple sclerosis, and type 1 diabetes. The company’s Disease Interception Accelerator group will search for…
Accounting for Portfolio Risk: Creating an Analytical Framework for Optimizing the Portfolio Mix
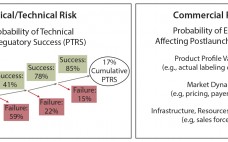
In the biopharmaceutical industry, portfolio risk is categorized as clinical/technical or commercial (Figure 1). Both types pose challenges to quantify. Portfolio managers tasked with optimizing the mix of products in a company’s pipeline often struggle to create apples-to-apples frameworks that can consistently compare risk across asset categories. The framework described herein can help you account for both technical and commercial risk using simple analytical tools. The foundational analytical element of portfolio management is traditionally the asset forecast: a composite best…
From Silos to Synergy: Optimize Your Managed-Markets Division with Cross-Team Collaboration
Like many other industries, life sciences faces rapid, technology-driven changes and a shifting business environment. Many patients are covered by Medicare, Medicaid, or national healthcare organizations outside the United States. That necessitates pricing and reimbursement negotiations that are complicated by rules and regulations. Patients whose health plans belong under a managed-care organization (MCO) or pharmacy-benefit manager (PBM) have access to prescriptions primarily through staff-model pharmacies, mail-order pharmacies, and network pharmacies. Drug manufacturers offer incentives to such organizations in the form…
Standardization of Disposables Design: The Path Forward for a Potential Game Changer
Recent articles have described how the debate on standardization is slowing down adoption of single-use technology (1). The Standardized Disposables Design (SDD) initiative is working to design simple standard single-use solutions for real-life examples (e.g., buffer bags). In reality, a buffer is a buffer whether it is made in Europe, Asia, or America, so in essence different solutions are not necessary for different end users. A buffer bag is not difficult to design, and it does not vary greatly in…
From Chips to CHO Cells: IT Advances in Upstream Bioprocessing
Advances in our capabilities for data acquisition, storage, and manipulation are providing the biopharmaceutical industry with an increased understanding of what must be controlled in bioproduction as well as the ability to control it. Developments in hardware, processing algorithms, and software are changing the landscape of bioprocess administration. Increased power for information gathering and processing began with the remarkable increases in microprocessor speed, pipelining, and parallelism over the past couple of decades (1); it continues with advances in data handling…
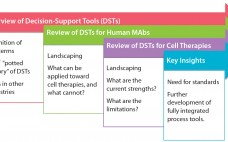
Decision-Support Tools for Monoclonal Antibody and Cell Therapy Bioprocessing: Current Landscape and Development Opportunities
Industrial-scale manufacturers in a number of fields — from automobiles to biotherapeutics — have long relied on powerful computational and mathematical tools to aid in the scale-up, optimization, quality control, and monitoring of product development (1–5). Typical process pathways are highly multifactorial, with numerous branch points, feedback steps, instrumental attributes, and target parameters. Moreover, margins for error are minimal for most industrial processes, requiring high standards of precision from industrial and operational pathways (6). For those reasons, the complexity of…
Primary and Higher-Order Structural Characterization Strategy for Biosimilarity Assessment
The development pathway of a biosimilar is unlike that of a novel biotherapeutic. Many regulatory authorities reference a stepwise approach to establishing biosimilarity. Analytical requirements are greatly increased before a product enters clinical testing. Enhanced analytical efforts entail physical, chemical, and biological characterization of a biosimilar product compared with an originator reference product. Strategies at this early stage must include intensive characterization of multiple batches to determine variability of quality attributes. Here I address some issues involved in meeting regulatory…