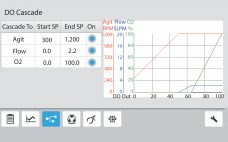
Learning to use a bioprocess controller is a complex endeavor. Even if someone has previous bioprocess experience, moving to a new software platform can entail much learning and reduce process efficiency. With the auto-culture modes of the Eppendorf BioFlo 120 bioprocess control station, a user can select with a push of a button either a predefined Escherichia coli batch fermentation protocol or a Chinese hamster ovary (CHO) batch cell culture process. Proof of Concept: E. coli Auto-Culture Fermentation The auto-culture…
Author Archives: Stacey S. Willard
Comparing Culture Methods in Monoclonal Antibody Production: Batch, Fed-Batch, and Perfusion
Recombinant protein manufacturing with Chinese hamster ovary (CHO) cells represents over 70% of the entire biopharmaceutical industry (1). In fact, human monoclonal antibodies (hMAbs) produced by CHO cells have played a major role in both the diagnostic and therapeutic markets for decades. One of the first human–mouse chimeric MAbs to obtain FDA approval was Roche’s rituximab treatment for non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and rheumatoid arthritis. Since that approval in 1997, scores of chimeric, humanized, and human MAbs have gained…