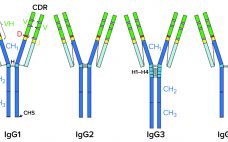
High–molecular-weight (HMW) and low–molecular-weight (LMW) product variants are critical quality attributes (CQAs) for monoclonal antibodies (MAbs) because they can cause severe immunogenic responses in human recipients. Aggregation is a common problem that can compromise the quality, efficacy, and safety of therapeutic proteins. It can occur at different stages in a biomanufacturing process: during cell-culture–based production, downstream process purification, drug-substance formulation, and storage of bulk drug substances or formulated drug products. Hence, the removal and control of MAb aggregates and fragments…
Author Archives: Saher Cheulkar
Removing Oligomers of a Recombinant Human Therapeutic Hormone:
Evaluation of Chromatographic Options for Effectiveness
Aggregation is a common cause of protein instability, which renders a biologic product unfit for therapeutic use. Sometimes it is difficult to purify monomeric proteins from oligomers because of similarities in their isoelectric points (pIs). Proteins such as hormones have pI ranges similar to their oligomers and thus can be difficult to separate out using a conventional polishing chromatographic step such as ion exchange. With those pI similarities, removal of oligomers to a considerable extent by ion exchangers can compromise…
Dye-Stripping Buffer and Resin Stripped-Dye Analysis: Development and Optimization of a Novel Spectrophotometric Assay and Method for Removal of Cibacron Blue Dye
Biopharmaceutical process-related impurities encompass all organic and inorganic materials that arise from the biomanufacturing process apart from the drug substance. According to ICH Q6B guidelines on test procedures and acceptance criteria for biotechnological products, these impurities include cell substrates such as host-cell proteins, host-cell DNA, endotoxins, and so on; inducers, antibiotics, media components, and chromatographic media used in purification processes; and solvents and buffer components (1). Impurities can cause undesired, deleterious pharmacological effects if ingested or injected by a patient,…
Dynamic Binding Capacities of Protein A Resins for Antibody Capture: A Comparative Evaluation
The dynamic binding capacity (DBC) of a chromatography resin represents the total amount of target protein that the resin will bind under actual flow conditions before significant breakthrough of unbound protein occurs. This is a useful parameter for predicting what the process performance of a resin will be in actual use. DBC affects the overall amount of resin that can be packed in a given column for a process — and the number of batches that can be processed cost-effectively…