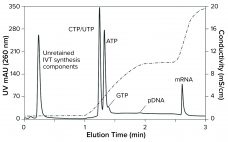
Rapid response to global pandemics requires the manufacture of billions of vaccine doses within months. This short timeline must allow for design and testing of active ingredients, development of production and purification processes, clinical evaluations, regulatory filings, and manufacturing. Existing purification methods often have been adopted from laboratory-scale techniques to allow rapid implementation, and those have provided adequate product quality. But future mRNA development will require optimized production and purification processes. Chromatography has been a workhorse of biomanufacturing for decades,…
Author Archives: Rok Sekirnik
Eliminating the Analytical Bottleneck in Production and Purification of mRNA
COVID-19 has focused a spotlight on the ability of mRNA technology to accelerate vaccine development and approval (1). That same technology can hasten development and approval of other therapeutic classes, including cancer immunotherapy, protein replacement, and gene therapy. Fulfilling those opportunities imposes significant challenges on process developers and manufacturers to improve existing processes. Scale-up to produce millions of doses (tens of kilograms) compounds those challenges. Furthermore, every step of the journey requires high-performance analytical methods, to ensure patient safety and…