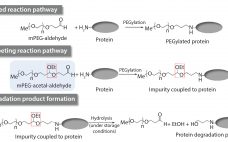
Impurities related to raw materials used for bioproduction can be inadvertently introduced into a manufacturing process, causing potential failure to meet in-process controls or release specifications. Unexpected impurities also can reduce yield and affect the quality, safety, and effectiveness of a final product (1). Raw-material impurities can originate from starting components or reagents used in manufacture. They can be generated in situ during synthesis or as degradation products. Impurities also can result from improper handling, packaging, and storage. Identification and…
Author Archives: Alejandro Toro
Changes in Raw Material Sources from Suppliers
Maintaining the supply chain of single-source raw materials is of utmost importance for a biopharmaceutical company’s manufacturing operations. As often happens, a supplier will notify its customer of process changes that might affect the quality or properties of supplied materials. Occasionally, a supplier might notify the customer of substitutions in its own supply chain or other changes in the source of its own raw materials. Customers must conduct appropriate testing using the “new” raw material(s) to ensure acceptable…