Biotechnology has enabled commercialization of protein-based drugs including insulin, growth factors, blood factors, and antibodies. Production and purification of such biologic products require different buffers for pH control and stabilization of reactions in different steps during biomanufacture.
These processes include cell culture production (the “upstream” phase), purification (the “downstream” phase), and a final phase in which excipients are introduced to the drug substance to create a drug product (“formulation and storage”). In upstream processes, buffers are primarily used for their capacity to help maintain culture pH within a specific range, optimizing conditions for cell growth and expansion, promoting production of a desired protein, and maintaining that protein’s functional characteristics during primary recovery before purification.
In downstream processing, buffers maintain defined purification conditions, control a protein’s ionization state as necessary for column chromatography, and stabilize the protein while maintaining its functional characteristics. For formulation and storage, buffers ensure optimal biological activity and stability of a drug product at the targeted storage temperature over the duration required for clinical or commercial use.
Buffers for Biomanufacturing
In selecting a buffer, it is important to consider the function and composition of the buffering solution needed for the specific stage of your process. A single step in purification can include several different buffer compositions, depending on the complexity of operation. Additional buffers are used in equipment equilibration and cleaning. When buffers are included to maintain a stable pH, other components such as salt, reducing agents, and/or stabilizers (e.g., polyols or detergents) to prevent protein aggregation are added once a suitable pH for protein stability and solubility has been determined.
To prepare solutions needed for process operations, buffer components and other dry additives are commonly added to water in powder form. Chemical compatibility must be demonstrated for such components with each other as well as the environmental conditions to which they will be exposed (e.g., temperature changes, light exposure). Additional factors to be considered are temperature shifts that can affect pH and buffering capacity of some chemicals; compatibility with salts, detergents, or reducing agents used in particular unit operations; special storage conditions; and compatibility with analytical assays.
In 1972, Norman Good and Seikichi Isawa identified several characteristics that describe an ideal buffer for biological systems (1): pKa between 6 and 8, high solubility in water, minimal passage through biological membranes, minimal salt concentration and temperature effects, minimal interactions between buffer and reaction components, high chemical stability, minimal light absorption and ease of use and preparation. These characteristics become especially important when a process is scaled up from research to clinical and commercial-scale biomanufacturing, in which reliable production of large volumes becomes a high priority.
Scale-up of buffer preparation — as described by Aldington and Bonnerjea (4) — can require adaptation. In laboratories, many buffers often are prepared based on volume; however, at larger scale, buffer preparation often is based on weight. Adjustments require consideration of buffer density. Buffers used in manufacturing should be formulated by combining acid and conjugate base forms rather than using exclusively one or the other and adjusting pH through gross titration with HCl or NaOH. Buffering capacity can be defined accurately using acid and conjugate base forms together at a predetermined ratio, and the pH of the resulting solution will be very close to target, requiring little to no additional adjustment (5). This method has been referred to as the preparation of “magic” buffers.
Although (hydroxymethyl)-aminomethane (Tris) is not one of the buffers described by Good and Isawa, it shares many of their identified ideal buffer characteristics and has become an essential buffer for manufacturing biologics. First produced commercially by ANGUS Chemical Company in 1953 through batch manufacturing (later by a patented continuous process beginning in 1967), Tris provides chemical stabilization to aqueous solutions in biomolecule purification. A buffer solution containing Tris base and Tris-HCl (its hydrochloride salt) at a molar ratio of 1:3 is considered standard for use in many biological systems.
Buffers for Drug Stabilization
Buffer components also can be useful as counterions in stabilization of small-molecule drug products. The weak acid or weak base components that make up a buffer can act as counterions to create salt formulations for drug molecules that themselves behave as weak acids or bases in aqueous solutions. The use of a weak acid or base as a counterion for drug molecules is referred to as saltification.
In 2008, Bansal et al. estimated that ~50% of organic compounds used as therapeutics in medicine are formulated as salts (2). Often behaving as weak acids or bases, such molecules can undergo varying degrees of ionization upon their dissolution in aqueous solutions. Saltification of a drug product that behaves as a weak acid or weak base often imparts characteristics such as greater water solubility, lower hygroscopicity, improved chemical stability, and better absorption (3). Using a weak acid or conjugate base component of a buffer for this purpose requires good manufacturing practice (GMP) certified chemicals of the highest quality and purity.
Identifying Buffer Specifications:Â Risk Assessment
Selection of a commercial buffer-component supplier for large-scale operations requires careful consideration on the part of the biomanufacturer. Before selecting a supplier, the manufacturer must perform a thorough QC assessment to determine necessary quality specifications based on the material’s use in its biomanufacturing process. A potential supplier should submit samples for initial chemical analysis by the company’s QC department. In addition, as part of its quality management system, the biomanufacturer’s QC department should monitor critical raw materials such as buffer components continuously to ensure that they reliably meet or exceed quality standards. Consistency and traceability of all raw materials must be established. Once a buffer is deemed suitable for a given biologic process, cost and ease of preparation become essential considerations for scaling up.
Risk analyses performed by qualified individuals are necessary for choosing the most appropriate grade of chemical for a specific application in biomanufacturing. The “Chemical Grades” box lists the different grades of chemicals and buffers available for biologic processes and laboratory research. As mentioned above, material grade can affect overall costs significantly as well as influence quality and consistency of the products they are used to make. Additional grades of chemicals — including industrial grade, laboratory grade, and technical grade — also are available. But because of inadequate control of impurities, they are not to be used in GMP manufacturing for pharmaceutical production.
| Chemical Grades |
| These are the basic chemical grades commonly used in biologic processes, research, and industrial chemical synthesis. |
| American Chemical Society: Meets or exceeds purity standards set by the American Chemical Society (ACS) |
| Multicompendial/Pharmacopoeial: With full compendial testing; meets or exceeds criteria defined by the BP, CP, DAB, JP, and PhEur |
| Reagent: Purity generally equal to ACS grade and suitable for laboratory and analytical applications |
| USP: Meets or exceeds requirements of the USP–NF and is acceptable for food, drug, and medicinal use |
| Abbreviations Used Herein |
| ACS: American Chemical Society
API: active pharmaceutical ingredient APIC: Active Pharmaceutical Ingredients Committee BCP: business continuity plan BP: British Pharmacopoeia BRIC: Brazil, Russia, India, and China CMR: carcinogenic, mutagenic, and reprotoxicological CP: Chinese Pharmacopoeia DAB: Deutsche Artsenei Buch ECHA: European Chemicals Agency HCl: hydrogen chloride ICH: International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use JP: Japanese Pharmacopoeia NaOH: sodium hydroxide PhEur: European Pharmacopoeia (EP) QC: quality control REACH: Registration, Evaluation, Authorisation, and Restriction of Chemicals TAUP: Tris(hydroxymethyl)aminomethane ultrapure THNM: Tris(hydroxymethyl)nitromethane Tris: tris(hydroxymethyl)-aminomethane USP-NF: US Pharmacopeia–National Formulary |
Choosing a Buffer Component Supplier
Using Risk Management to Set Up a Supplier Qualification System: The ICH Q9 document specifies that quality risk management should be implemented such that “evaluation of the risk to quality should be based on scientific knowledge and ultimately link to the protection of the patient. The level of effort, formality and documentation of the Quality Risk Management process should be commensurate with the level of risk” (6). This guideline forms the basis of supplier selection processes for biomanufacturers.
A risk management system can be effective only with an adequate scope of information. The range of information that a biomanufacturer collects, retains, and monitors from its suppliers should be based upon the “critical” nature of each raw material in a given process. Use of buffers in upstream and downstream bioprocessing makes them critical process components. But their apparent widespread availability from multiple suppliers often lowers the priority of their sourcing and approval procedures compared with single-sourced raw materials. Here, we offer an example of the role that complex factors can play in the sourcing of the widely used and available buffer component Tris.
Designing a Supplier Approval Procedure: Each biomanufacturer should have and follow a supplier approval procedure. To prepare for supplier selection, the company first must ensure that the buffer components being sourced meet predefined specifications identified by a thorough QC assessment. That assessment should be based upon each material’s use in a given manufacturing process. Potential suppliers should submit samples for initial chemical analysis.
Regulatory agencies can and will audit biomanufacturers. And although they can and sometimes do audit vendors, regulatory agencies expect that biomanufacturers will independently approve and monitor their own suppliers. A well-documented supplier approval procedure can verify that a biomanufacturer has taken appropriate steps in its initial selection process and that self-auditing methods are in place to ensure control of the manufacturing process at all levels of supply management.
In 2009, the Active Pharmaceutical Ingredients Committee (APIC) of the European Chemical Industry Council issued a document that includes a list of five key criteria for selection of raw material suppliers (7): supply assurance, compliance, cost, supplier technical expertise, and responsiveness. As environmental regulations for toxic chemical use continue to evolve and become more stringent, we suggest considering environmental responsibility and sustainable manufacturing as criteria for supplier selection as well.
Cost: Although the cost of a critical raw material is an important criterion, the benefit of obtaining raw materials at lower cost must be weighed against the other supplier selection criteria listed above. Production costs never should be minimized by compromising the primary objectives: quality and safety of the final product.
Process modeling can be used to estimate the direct cost of buffer components associated with a biomanufacturing process. Indeed, in anticipation of a possible supplier change for a given raw material, the need for change control will increase its cost overall. Designating and preapproving an alternative supplier will minimize change-control costs, as can an approved supplier with multiple independent production facilities. Because buffer components are readily available, alternative suppliers can be considered fairly easily. If the chosen supplier is a distributor rather than the component’s primary producer, it is important that the alternative supplier and primary supplier do not use the same source manufacturer for their supplied raw materials.
Supply Assurance and Compliance: A stable and reliable supply chain for raw materials is vitally important to all phases of a product’s lifecycle, from early development to full-scale manufacturing. Inconsistencies in the supply of a particular buffer component — or modifications to the supplier’s manufacturing process — will require biomanufacturers to assess potential implications on overall product quality (and perhaps to consider changing suppliers). Change control increases production costs through additional risk assessments, repeat sourcing of buffer components, and verifying new supplies.
Supply assurance is based on supplier consistency in quality and logistics, thorough contingency planning, and adaptability. To maintain an assurance of raw material supply, suppliers must have sufficient production capacity to produce a given buffer component reliably in accordance with specifications defined by appropriate quality unit(s), in needed quantities, and within required time frames. Moreover, their logistics systems should allow them to monitor, record, and maintain delivery conditions (as necessary) to ensure high quality of these raw materials (8).
Determining whether a supplier has a business continuity plan (BCP) in place may help prevent potential loss of supply in the case of unexpected or unlikely events. Engaging suppliers that have more than one manufacturing location — or secondary suppliers prequalified by QC — helps companies prevent potential sourcing crises. A 2014 case study examined issues in supply management following the 2011 tsunami in Japan (9). It describes the use of BCPs by an active pharmaceutical ingredient (API) manufacturer and identifies key themes in supplier BCPs.
The ability of a supplier to produce buffer components for use in GMP manufacturing also depends on its environmental compliance and ability to adapt quickly to evolving policies. Because of their low labor and production costs, emerging economies (e.g., Brazil, Russia, India, and China — BRIC) are major producers of chemicals on the current worldwide market. However, environmental policy in those countries has been slow to catch up to rapid expansion of the global market for chemicals.
China has undergone rapid evolution in its environmental policies because of increasing public concern over the extent of pollution in the air, soil, and water supplies of its industrialized regions. The Chinese government amended its Environmental Protection Law in 2014 and 2015, and the Chinese Ministry of Environmental Protection implemented new water and soil pollution regulations and started the process of centralizing environmental oversight.
To limit chemical exposure in more densely populated areas of China, many chemical plants have been relocated to remote industrial parks, each housing several manufacturers. By the end of 2011, more than 5,600 Chinese chemical plants were relocated to newly developed industrial parks to facilitate safer hazardous waste management and disposal (10).
Unexpectedly, that relocation of chemical manufacturers contributed to a recent significant disruption in the Tris buffer supply. In April 2016, students at a school in Jiangsu province — near the site of a newly developed industrial park, the Jiangsu Economic Development Zone — began experiencing mysterious rashes and nosebleeds (11). Close proximity of many potential effluent sources made determining the source of chemical contamination difficult, and local government officials forced the temporary closure of several chemical plants in the vicinity until the source of the problem was isolated.
Chemical plants that manufacture Tris and raw materials required for its production were among those located in the targeted Jiangsu Economic Development Zone. Although these particular plants were not implicated as pollution sources, they were closed until the formal investigation could be completed. Those closures caused chemical shortages that affected multiple steps in the Tris manufacturing process and led to a shortfall in the global supply of Tris that left distributors and direct suppliers (companies that purchase Tris crystals) temporarily unable to source the material.
Although buffer components such as Tris are considered “readily available,” it may be surprising to many people working in biologics production that the number of primary chemical production facilities that produce Tris actually is quite limited. Currently three companies serve as primary producers of Tris worldwide: two located in China and ANGUS Chemical Company in the European Union and United States. Many distributors that depended on facilities affected by the Jiangsu closure mandate in China had no contingency plans to deal with a temporary halt in production. Consequently, the shortages caused a cascade effect across many suppliers and distributors. With in-house production of all intermediates, ANGUS was able to fill the Tris supply void, thereby limiting the worldwide impact of the disruption.
As China’s environmental policy evolves, chemical manufacturers must be able to adapt quickly to the new regulations regarding hazardous chemical handling and waste management. That will help them to maintain safety conditions and meet market demand.
Established markets are not exempt from evolving environmental policy, however. In 2008, the European Chemicals Agency (ECHA) adopted its Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) regulations that require all companies manufacturing or importing chemicals in quantities greater than a metric ton per year to register those chemicals, perform risk assessments, and demonstrate measures taken to minimize risk (12).
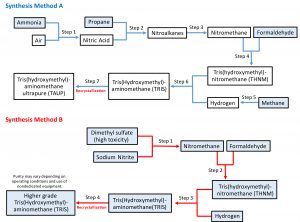
Figure 1: There are two main methods of synthesis used by major Tris producers. Both produce nitromethane and THMN. Process A synthesizes nitromethane from nitroalkanes and uses a continuous in-house manufacturing process. Process B uses dimethyl sulfate and sodium nitrite to synthesize nitromethane. Direct suppliers also can purchase Tris crystals to wash and recrystallize and generate a higher-purity grade of Tris. Boxes highlighted in blue are chemicals used as starting materials for synthesis or that can be purchased from raw materials suppliers for Tris production.
REACH regulations could play a role in the methods of production for certain chemicals in Europe. For example, currently two principal methods of Tris synthesis are in commercial use (Figure 1); both producing nitromethane and THMN. One process uses ammonia, propane, formaldehyde, and methane sourced from suppliers (Figure 1, top). ANGUS Chemical Company operates this process for its fully in-house Tris production.
The second synthetic method (Figure 1, bottom) uses dimethyl sulfate and sodium nitrate as starting materials. For some manufacturers, this is a discontinuous process distributed across multiple producers that are themselves sourcing intermediates from different suppliers for THMN production. This method of synthesis is not used in either the United States or Europe. Dimethyl sulfate is classified by the European Union as a “substance of very high concern due to its carcinogenic, mutagenic and reprotoxicological (CMR) properties” (13). An alternative method of production (not requiring chemical synthesis) is used is used by direct suppliers. Such companies purchase Tris crystals, then simply wash and recrystallize them to generate their own grades of Tris.
The synthetic process depicted in the top panel of Figure 1 both reduces the use of highly toxic compounds and eliminates potential incorporation of toxic impurities associated with nitromethane produced by the wet-chemical dimethyl sulfate method. ANGUS produces a pharmaceutical-grade nitromethane and controls the manufacturing of all key intermediates by producing them in-house. Manufacturing waste is treated and disposed of onsite at its facilities in Louisiana and Germany.
EU regulations state, “Where possible, starting materials should be purchased directly from the manufacturer of the starting material” (14). Those GMP guidelines request traceability of chemical products to their original manufacturers. The ability to access the provenance of chemical components used in manufacturing buffer materials can assist in auditing procedures and better ensure compliance in raw material sourcing. If a chemical company manufactures key raw materials used in the Tris manufacturing process as well as the final product itself, then users of that final product can be assured that its synthetic intermediates are 100% traceable.
Manufacturing Expertise: Technical expertise among suppliers of raw materials is often undervalued by their customers. This includes innovation in chemical synthesis and manufacturing technology. That is important to ensure a supplier’s ability to tailor its buffer component’s chemical specifications to fit the requirements dictated by quality management. It also allows the company to modify manufacturing techniques to fit regulatory changes.
Chemical synthesis of buffer components such as Tris is limited to a handful of producers. Most suppliers purchase raw Tris crystals from chemical producers and recrystallize them to generate additional purity grades (Figure 1). When dealing in large bulk amounts of buffer components, primary manufacturers can adjust their manufacturing or analytical processes to meet specific customer requirements, whereas suppliers providing recrystallized Tris are not as flexible.
Responsiveness: Supply assurance requires good communication between vendor and customer. Quality agreements and supply contracts are just two interactions that must take place between them. Regulatory auditors can request information from suppliers to survey their raw material supply chains. A supplier’s ability and willingness to provide necessary documentation readily can save biomanufacturers both time and cost. Buffer component suppliers — both distributors and manufacturers — should be able to provide product specifications, manufacturing and packaging details, safety data sheets (SDSs), and quality systems certificates readily. They need to identify and describe product supply logistics and analytical test methods. Distributors and manufacturers also should be able to supply auditors with information regarding raw materials.
Postselection Testing and Final Approvals: Once a supplier has been identified and shortlisted, a biomanufacturer should negotiate quality and supply contracts to ensure a consistent and specific quality designation for all buffer components. Such contracts should cover GMP manufacturing conditions, compendial status, trace-element concentrations, microbiological control, and other purity considerations. Supply agreements also should be negotiated for buffer components because of the significant amounts used in downstream processing.
GMP guidelines dictate that a quality system is required for identification, selection, and approval of all materials in API manufacturing. Supplier selection should include sample evaluation and, for critical raw materials, production trials.
Critical Components Are Serious Business
Selecting the correct buffers for bioprocessing requires careful consideration of many variables: process–component compatibility, functionality and buffering capacity at process temperatures, cost, and ease of use at large scale. Important criteria to consider are traceability and transparency of raw material in supply chains, which directly affect manufacturing consistency. Suppliers with whom a biomanufacturer can build reliable supply relationships are key to successful outcomes for its own products. Vetting the full scope of a supplier’s capability is an essential step to ensuring that success.
References
1 Good NE, Izawa S. Hydrogen Ion Buffers. Meth. Enzymol. Photosynth. Nitrogen Fix. Part B. 24, 1972: 53–68.
2 Bansal AK, Kumar L, Amin A. Salt Selection in Drug Development. Pharm. Tech. 32(3) 2008.
3 Ohtake S, Kita Y, Arakawa T. Interactions of Formulation Excipients with Proteins in Solution and in the Dried State. Adv. Drug. Deliv. Rev. 63(13) 2011: 1053–1073.
4 Aldington S, Bonnerjea J. Scale-Up of Monoclonal Antibody Purification Processes. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 848(1) 2007: 64–78.
5 Perrin DD, Dempsey B. Buffers for pH and Metal Ion Control. Wiley: New York, NY, 1974.
6 ICH Q9: Quality Risk Management. International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, November 2005; www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q9/Step4/Q9_Guideline.pdf.
7 Supplier Qualification and Management Guideline. Active Pharmaceutical Ingredients Committee: Brussels, Belgium, December 2009; http://apic.cefic.org/pub/guidelinesupplierqualification_200912_final.pdf.
8 ICH Q7A: Good Manufacturing Practice Guide for Active Pharmaceutical Ingredients. International Council on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH): Geneva, Switzerland, November 2000; www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q7/Step4/Q7_Guideline.pdf.
9 Jagschies G, Westman D, Raw D. Supply Chain Challenges in the Biopharmaceutical Industry: A Case Study Following the 2011 Tsunami in Japan. BioProcess Int. 12(8) 2014: 18–27.
10 Fu D, Shi Y. Chemical Company Linked to Toxic School Has Tainted Past: A Cavalier Attitude to Handling Dangerous Materials Comes Back to Bite China’s Chemical Industry. Sixth Tone 23 April 2016.
11 Chemical Plants Shut After Kids Suffer Rashes. Straits Times 21 April 2016.
12 Regulation for Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) Guidelines. European Chemicals Agency: Helsinki, Finland, 2008; http://echa.europa.eu/guidance-documents/guidance-on-reach.
13 SVHC Support Document: Dimethyl Sulphate. European Chemicals Agency: Helsinki, Finland, 2008; https://echa.europa.eu/documents/10162/8fcaf1f0-ea6d-4ec8-a129-f0405def7c33.
14 Chapter One: Pharmaceutical Quality System (Revision 3). EudraLex: Rules Governing Medicinal Products in the European Union, Volume 4 — Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. European Commission Health and Consumers Directorate-General: Brussels, Belgium, January 2013: 1–8; http://ec.europa.eu/health//sites/health/files/files/eudralex/vol-4/vol4-chap1_2012-06_en.pdf.
Jennifer Bratt and Angela Linderholm are consultants, and corresponding author Steven M. Chamow is principal consultant at Chamow & Associates, Inc. in San Mateo, CA; 1-650-345-1878; steve@chamowassociates.com. G. David Green is vice president of research and development at ANGUS Chemical Company in Buffalo Grove, IL.

