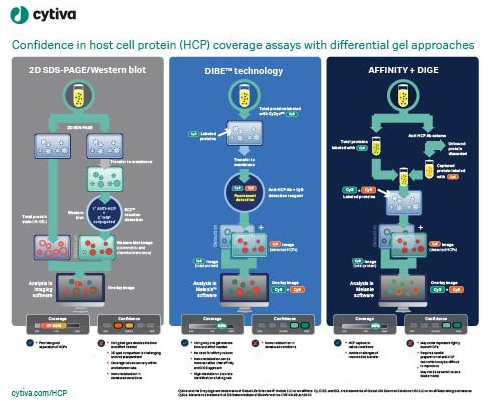
Enzyme-linked immunosorbent assays (ELISAs) are critical to detecting and removing host cell proteins (HCPs), a primary source of impurities in biologics development. Scientists must validate HCP ELISAs to ensure patient safety and meet regulatory guidelines – but different coverage assays come with different challenges, limitations, and benefits. The US Pharmacopeia recommends 2D differential gel electrophoresis (DIGE) combined with Western blot or immunoaffinity approaches, and labs might not be able to gain the full benefits of DIGE without significantly altering current procedures. Understanding the options available can help scientists implement a coverage strategy that optimizes accuracy and minimizes the risk of delay in the biologic approval process.
This visual guide summarizes the steps involved in differential gel approaches and immunodetections, outlines their strengths and weaknesses, and compares the level of confidence they can provide to your processes. A clear and comprehensive view of the facts can help you make an informed decision to enhance speed and accuracy throughout biologics development and manufacture.
