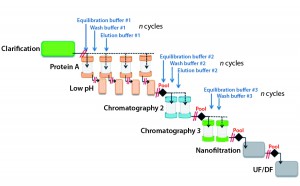
Process intensification through continuous manufacturing has been practiced in the chemical, petrochemical, and food industries for years and has gained much interest among biopharmaceutical manufacturers (1). Key drivers encouraging biomanufacturers of therapeutic molecules to convert batch processes into continuous operation include flexibility, productivity, cost effectiveness, and product consistency.
Continuous upstream processing has been demonstrated for the manufacture of a broad range of molecules, including complex/labile proteins such as enzymes (2) and monoclonal antibodies (3). Recent publications have reported successful application of single-use technologies for perfusion processes as a next logical development step in combining flexibility with process efficiency (4).
For continuous downstream processing, however, additional efforts will be required to attain a similar level of maturity and finally provide for robust continuous unit operations. Current concepts for process intensification in downstream processing mostly involve continuous chromatography through multicolumn approaches. Such concepts are based on connecting multiple columns packed with the same media in series to optimize resin use up to maximum capacity during the loading phase. The required number of serially connected columns depends on upstream process titers and targeted residence times. Better capacity use and reduction of residence time can considerably improve productivity and provide savings on resins and buffer consumption. That is especially beneficial when expensive chromatography resins are used (e.g., protein A affinity capture). An associated reduction in column size is particularly relevant for clinical manufacturing, in which relatively large columns often are used for just a few cycles to meet process time requirements.
Even though such modes of operation can provide economic benefits, some limitations remain for their implementation at larger manufacturing scales. Complexity is often considered to be a remaining barrier when four or more columns are required, thereby necessitating a more convoluted valve arrangement. Column variability is another consideration, especially with regard to packing and operating robustness.
Continuous Multistep Chromatography
An alternative manufacturing strategy has been presented recently for overcoming such operational complications and achieving higher downstream process productivities. Accelerated seamless antibody purification (the ASAP approach) was developed at Sanofi in France as a simplified continuous-purification strategy to increase process efficiency. This mode of operation has been evaluated and implemented under good manufacturing practice (GMP) conditions at 500-L scale to produce 1.3 kg of monoclonal antibody (MAb). A 66% reduction in column size was achieved along with a 10-fold increase in productivity.
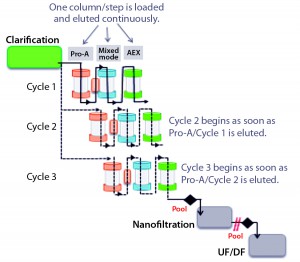
This purification process is based on three industry-proven chromatographic steps. The first is a high-efficiency protein A capture step. The second step can use either a cation-exchange (CEX) or mixed-mode ligand, the latter of which interacts with protein targets through several mechanisms (e.g., ionic, hydrophobic, and hydrogen-bond chemistries). The third step is flow-through polishing chromatography using an anion-exchange resin. What differentiates the ASAP approach from classical batch operations is that all chromatographic steps are performed continuously without intermediate holding times or process-fluid adjustments. Each unit operation can be directly connected to the subsequent step, thereby simplifying the overall downstream process.
In detail, eluate from the capture step either can be loaded directly onto a second-step column or undergo an in-line virus-inactivation procedure first, depending on the molecule. Eluate from the second step is applied directly onto the polishing column, then ultimately recovered through a sterilizing-grade filter. Therefore, a key benefit of ASAP continuous processing is its elimination of “non–value-added” unit operations such as adjustment of pH, molarity, and protein concentration; intermediate filtration; and storage of process intermediates. With those steps removed, process cycle times and column sizes can be reduced while productivity is enhanced.
Such a simplified operation allows for fast processing of a complete three-step chromatographic process, and combined with a multiple-cycle strategy, increases production capacity. Furthermore, rather than each unit operation being repeated and pooled before processing moves forward, all steps are performed simultaneously and continuously (Figure 1). As soon as the eluate of one column is transferred to the next step, the former can be regenerated, equilibrated, and loaded again — and likewise for the second- and third-step columns. The process requires only one purification skid, with three independently operated pumps to drive all steps at the same time. As a consequence of that tailored automation, overall processing time can be drastically reduced.
The ASAP design further simplifies MAb purification through using only four buffers for the whole operation. Compare that with the minimum of nine buffers required by a classical three-step process. To facilitate preparation, all buffers are made with the same components. Because one ASAP purification cycle is similar to a classical batch-operation sequence, moving from batch to continuous mode — or even from continuous to batch mode — requires no specific conversion or additional process development activities.
Membrane Adsorbers Offer an Alternative
To demonstrate the potential for moving toward a fully disposable manufacturing platform through the ASAP concept, we replaced traditional chromatography steps with single-use membrane adsorber technology. Because of improved hydrodynamics, membrane adsorbers typically can be operated at 10× to 50× higher flowrates than traditional resins packed in columns. Thus, purification can be achieved in a significantly smaller operating footprint without lessening productivity. Users can expect lower variability among these devices because no packing is required, and bed height is a function of the number of membrane layers present. These products come in a broad range of chemistries, scales, and bed heights to simplify accurate sizing of the three steps described herein for adsorptive capacity and speed within the ASAP concept. Recent improvements in capsule design — especially regarding void-volume reduction — have extended the applicability of this technology for bind-and-elute (BE) chromatography and further increased membrane-adsorber versatility.
In our study, we first evaluated the suitability of membrane-adsorber technology in a three-step disposable process for continuous and multiple-cycle operations at laboratory scale. Next, we demonstrated the scalability of this approach at the 50-L scale and assessed its productivity and impurity removal capability. Finally, we modeled the process and performed an economic comparison of an ASAP process based on membrane adsorbers with a traditional resin-based column process, showing the cost effectiveness of the membrane-adsorber–based approach.

Figure 2: Overview of membrane chromatography capsule portfolio with bed volumes ranging from 3 mL to 5 L and from 1 mL to 2.5 L, with 4-mm and 8-mm bed heights, respectively (left); cutaway view of a 150-mL capsule with 8-mm bed height (right) with blue and red flow channels
Materials and Methods
Clarified Cell Culture Fluid (CCCF)/Bulk Harvest: To produce a fully human immunoglobulin G (IgG) antibody, we cultured Chinese hamster ovary (CHO) cells in bioreactors using chemically defined media and feeds. We removed cells from their culture suspension with body-feed filtration, then filtered the supernatant with a 0.2-µm grade filter before storing it at 2–8 °C until needed.
Chromatography Media and Systems: All membrane adsorbers came from Sartorius Stedim Biotech. For both laboratory- and pilot-scale experiments, we used Sartobind protein A 2-mL and 70-mL, Sartobind S 3-mL and 150-mL, and Sartobind Q 3-mL and 75-mL devices. ÄKTA Pure and ÄKTA PCC chromatographic systems from GE Healthcare hosted the lab-scale experiments. For pilot-scale evaluation, we used ÄKTA Process systems, also from GE Healthcare.
Lab-Scale Single-Use MAb Purification Process: Our single-use laboratory-scale purification process used Sartobind protein A 2-mL, Sartobind S 3-mL, and Sartobind Q 3-mL adsorbers. We evaluated antibody recovery, quality, and purity from the eluate of each step as described below.
Analytics: To determine antibody concentration we used UV absorbance measurement at 280 nm on a Nanovue instrument from GE Healthcare. To determine high–molecular-weight (HMW) and low–molecular-weight (LMW) content, we used sizeexclusion high-performance liquid-chromatography (SE-HPLC) on a Waters SEC-HPLC instrument. We evaluated host-cell protein (HCP) contamination by enzyme-linked immunosorbent assay (ELISA), and DNA content by reverse-transcription quantitative polymerase chain reaction (RT-QPCR).
Small-Scale, Proof-of-Concept, Fully Disposable Continuous Process with Membrane Adsorbers: To achieve a three-step, fully disposable continuous process, we connected four Sartobind protein A 2-mL columns in a 2 + 2 configuration such that two streams would run in parallel with two columns in series for each stream. We operated two Sartobind S 3-mL in parallel and completed the process with a single Sartobind Q 3-mL device. Each membrane adsorber was prepared as recommended by Sartorius Stedim Biotech (Sartobind manual, order number 85037-549-25).
Pilot-Scale Single-Use ASAP Process: Each cycle of the purification scheme was completed as follows:
- The capture step used two Sartobind protein A 70-mL devices connected in parallel to provide sufficient binding capacity and achieve the required flow.
- For each cycle, 840 mg of MAb was loaded onto the membrane, corresponding with a loading capacity of 6 g/L of membrane.
- Eluate from the protein A adsorber was directly transferred onto a 150-mL Sartobind S device. Eluate of this second step was passed through a Sartobind Q 75-mL membrane.
- Product from each cycle was recovered, sterile-filtered, and added to the collection bag. All cycles are completed by a dedicated cleaning procedure performed independently on each device before the process repeated.
- A flow rate of >60-L/h was used and equivalent to 6–15 membrane volumes per minute, depending on the size of the device. Residence times were six to nine seconds.
We used two Ă„KTA process systems, one of which was dedicated to the protein A capture step and the other for the two ion-exchange adsorbers. We ran these systems independently, but the whole process was continuous without intervention.
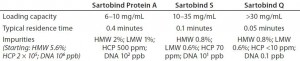
Table 1: Average performance of membrane adsorbers; binding capacity, residence time, and impurity levels measured after each chromatographic step within one ASAP cycle
Results: Proof of Concept
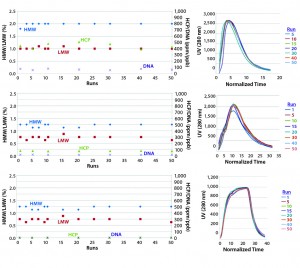
Feasibility and Performance of a Fully Single-Use ASAP Concept at Laboratory Scale: In the first stage, our objective was to implement and evaluate membrane adsorbers in the ASAP processing platform as an alternative to classical resin-based media packed into columns for MAb purification. We analyzed purification performance and compared the results with those of packed-bed chromatography standards. Table 1 shows comparable recovery data as well as aggregate and contaminant removal for both approaches. Because of the intrinsically high flow performance of membranes, one complete purification cycle took <15 minutes. A disposable process using membrane adsorbers could provide efficient purification with the same performance as industry-standard resin technology. Binding capacities were lower for membrane adsorbers than for traditional resin–based media, but those capacities were achieved at a much lower residence time and proved to be independent of the flow rate (data not shown).
Reusability and Robustness of Membrane Adsorber Technology within ASAP Concept: Membrane adsorber chromatographic devices are mainly used in MAb purification processes as a single-use technology for the removal of contaminants including DNA, HCPs, and viruses and are typically discarded after use.
The ASAP concept relies on process intensification and productivity increases through fast processing, column volume reduction and multiple-cycle use. Because the Sartobind membrane adsorber technology is based on crosslinked cellulose with high chemical stability, the capability of such disposable devices to handle a large number of cycles with consistent performance was evaluated. This would allow the purification of large amounts of material using a low volume of membrane and with a number of cycles corresponding to the maximum lifetime of the media, thus opening the door for single-(batch)-use protein purification.
We evaluated Sartobind protein A membranes for multiple-cycle use. After optimization of the cleaning strategy, more than 300 runs could be performed without any increase in pressure. Beside the mechanical stability, the robustness and consistency of the technology was also evaluated. We monitored purification performance across all the cycles. Comparable elution peaks (UV shape), recovery yield, and contaminant profiles were observed in all samples (Figure 3) and proved to be consistent during the processing of the entire batch.
Results: Scalability
In the pilot-scale experiment, we evaluated process scalability at pilot scale using a 50-g batch of MAb. The complete purification operation took 2.6 hours, was fully automated, and required no equipment modifications. Our single-use ASAP process allowed a fully enclosed purification of the antibody from CCCF to final purified product without operator intervention.
Drug substance analysis showed efficient purification performance with a yield of >90%, a low aggregate content (<1%), and high purity (<10Â ppm HCPs). Expressed in terms of protein A volume, the achieved productivity was 125 g/L/h, corresponding to a 50-fold improvement over a classical resin-packed column process in batch mode. Full processing time, chromatography media requirements, and footprint were all drastically reduced. This shows disruptive potential for industrial MAb production.
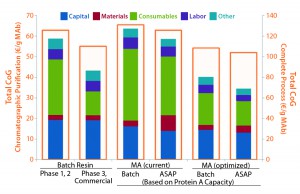
Figure 4: Comparison of different process scenarios shows cost per gram of monoclonal antibody (MAb) from chromatographic purification — protein A including virus inactivation, cation and anion exchange (CEX and AEX) — and for the overall manufacturing process. “Materials” and “Consumables” cover buffers and bags/chromatography media, respectively. Facility running costs and equipment costs are allocated in “Capital.” Current protein A capacity is 6 mg/mL, optimized protein membrane capacity is a theoretical binding capacity of 25 g/L.
Results: Cost Analysis
Economic Evaluation of Single-Use ASAP Concept: Using Biosolve Process 6 software from Biopharm Services, we evaluated process costs by considering six different scenarios: batch processing with resin-packed columns during phase 1 and 2; the same type of process during phase 3 and commercial manufacturing; batch processing with membrane adsorbers (MA) and current protein A capacity; the same with optimized capacity; and ASAP processing with membrane adsorbers at current and optimized protein A capacities. Using the determined binding capacities and the operating conditions above, we built our models assuming a 1,000-L batch with an antibody expression titer of 5 g/L. For comparison purposes, we assumed the costs involved for production and clarification before the capture step and after chromatographic purification — e.g., virus filtration and ultrafiltration/diafiltration (UF/DF) — to be identical in all considered processes.
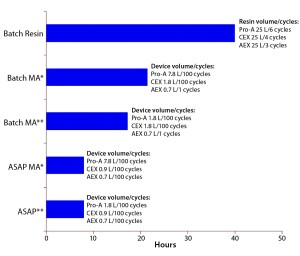
Figure 5: Summary of process duration and required chromatography media volume for different purification approaches to a 1,000-L batch
As mentioned above, we considered two different resin-based process cases: a phase 1–2 process in which only a fraction of the maximum resin lifetime is used, and a phase 3 and commercial process with full use of the maximum resin lifetime. The maximum lifetime of both resins and membrane adsorbers is 100 cycles. We chose a lifetime use of only 20 cycles for the resin phase 1–2 process. For all simulated membrane-adsorber–based processes (both batch and ASAP approaches), we assumed that 100 cycles would be achieved within one batch and sized the membrane adsorber devices accordingly (Figure 5). They would be discarded after each batch. By contrast, to allow for comparable column sizes, the respective number of cycles within resin-based processes were as follows:
- protein A — six cycles
- cation-exchange (CEX) — four cycles
- anion-exchange (AEX) — three cycles.
In a second evaluation, we considered an optimized membrane adsorber-based process (both batch and ASAP approaches) with a binding capacity of the first BE step (protein A) increased to 25 g/L. This would highlight the impact of performance for that particular chromatographic medium on overall cost of goods (CoG). Our cost analysis showed that a membrane-based process exhibits comparable CoG to a typical resin process in phase 1–2 with current protein A binding capacities, especially with the ASAP approach. Generally, membrane-based processes require less investment than resin-based processes because of the cost of column hardware.
Our cost simulation also suggests that an increased binding capacity of protein A membrane adsorbers from 6–10 g/L to 25 g/L would be required for them to compete on cost with commercial resin-based process. Associated cost savings are attributable not only to the lower required membrane adsorber volumes, but also the corresponding lower costs for buffer and bags.
In all simulated cases, ASAP purification processes were most productive; with increased protein A binding capacity, they are also the most cost efficient. This is mainly explained by a decrease in labor and consumables costs that come with a relatively short process duration and an absence of intermediate storage bags, respectively. The reduced capital costs for an ASAP process results from operating only a single chromatography skid for all chromatographic steps, with a correspondingly reduced need for surge tanks or bags.
Potentially Disruptive
Our study demonstrates the applicability of membrane-adsorber technology for new processing concepts such as the ASAP process developed by Sanofi. Very high volumetric flow rates can be achieved in a chromatographic purification train that uses only membrane adsorbers and no resins. As a consequence, the time requirements are reduced for complete chromatographic cycles, allowing for full use of the maximum membrane lifetime in each batch. Our experimental data illustrate that the gain in productivity obtained by moving from resin-packed columns to membrane adsorbers on one hand and by moving from batch to ASAP operations on the other can dramatically reduce both column/device size and processing times, as was confirmed by process simulation. Cost analysis identified the main costdriving parameters within a batch and ASAP operation mode as well as defining performance requirements for next-generation membrane adsorbers. High volumetric throughput in a small operating footprint suggests a disruptive potential for protein A membrane adsorbers.
Increased binding capacity for the capture step would significantly reduce the CoG here by lowering both consumable and buffer costs (chromatography media and bags), but they would remain comparable to those of traditional resin-based commercial DSP. For phase 1–2 clinical manufacturing, the advantages offered by membrane-adsorber technology are both economic and productivity-related because such a scenario typically uses only a fraction of the maximum lifetime of protein A resins.
Finally, implementation of membrane technology within an ASAP process provides for a single-use downstream process capable of yielding an entirely purified MAb in a few hours while reducing the volume of chromatography media used. This would considerably increase flexibility and downstream processing capacity in future facilities. The ability to process the complete output of one single-use bioreactor within one day — or even within one production shift — would further encourage implementation of single-use technology in commercial bioprocesses. It may also drive how facilities of the future will use their operating time by favoring an increased number of parallel single-use bioreactors for commercial production over large stainless steel bioreactors that are cleaned and reused.
References
1 Anderson N. Practical Use of Continuous Processing in Developing and Scaling Up Laboratory Processes. Org. Proc. Res. Dev. 5(6) 2001: 613–621; doi:10.1021/op0100605.
2 Warikoo V, et al. Integrated Continuous Production of Recombinant Therapeutic Proteins. Biotechnol. Bioeng. 109(12) 2012: 3018–3029; doi:10.1002/bit.24584.
3 Pollock J, Ho S, Farid S. Fed-Batch and Perfusion Culture Processes: Economic, Environmental, and Operational Feasibility Under Uncertainty. Biotechnol. Bioeng. 110(1) 2013: 206–219; doi:10.1002/bit.24608.
4 Martin J. Case Study: Continuous Processing at 2,000-L Single-Use Bioreactor Scale. 24th ESACT Conference (Barcelona, Spain). European Society for Animal Cell Technology: Frankfurt am Main, Germany, 2015.
Further reading
Process Intensification: Engineering for Efficiency, Sustainability and Flexibility. Reay D, Ramshaw C, Harvey A, Eds. Elsevier Ltd.: London, UK, 2008.
Tanner HA. Continuous Casting: A Revolution in Steel: the Worldwide Success Story of Comcast AG, ZĂĽrich. Write Stuff Enterprises: Fort Lauderdale, FL, 1998.
Corresponding author Benoit Mothes is head of scientific and downstream innovation, and Jerome Pezzini is a downstream process scientist, at Sanofi, Impasse des ateliers, 94400 Vitry Sur Seine, France; +33-155-71-0340; benoit.mothes@sanofi.com. Kathrin Schroeder-Tittmann is a scientist in membrane R&D, and Louis Villain is director of R&D for membrane modification at Sartorius Stedim Biotech in Göttingen, Germany.