
Membrane chromatography is an effective alternative to conventional beaded sorbents for the purification of large virus molecules or viral-like particles. Membranes have an open pore structure that allows fast kinetics and direct access to ion-exchange binding sites, with flow rates that are orders of magnitude faster than conventional columns. They provide much higher binding capacities than beaded sorbents for large molecules limited by mass transfer. Pall Mustang ion-exchange membranes are scalable membrane chromatography devices used in various therapeutic, vaccine, and gene therapy products purification schemes. These ready-to-use devices (reusable or single-use) allow users to significantly reduce hardware investments and validation costs typically associated with column chromatography.
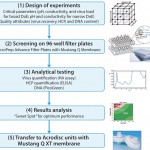
A study performed on live influenza (H1N1) showed how structured design of experiments (DoE) and high-throughput screening (HTS) experiments performed on AcroPrep Advance 96-well filter plates with Mustang Q membranes can be applied for fast and effective selection of purification conditions of influenza virus from clarified cell culture feedstock (1). The HTS approach (Figure 1) requires minimal sample consumption, and predicted results are in line with standard chromatography experiments directly obtained with Acrodisc® ion-exchange chromatography units with Mustang Q XT membrane.
Results
Selection of Bind/Elute Conditions by HTS on AcroPrep Advance 96-Well Filter Plate with Mustang Q Membrane Dynamic Binding Capacity (DBC) for Influenza Virus: The sweet spot of the initial DoE (Figure 2, down right) showed that a high DBC between 1.10 to 1.15 x 105 HA units/mL of membrane (4.1 to 4.5 x 1011particles/mL of membrane) can be reached. Good HCP clearance was achieved (HCP remain mostly in the flow through), whereas no DNA clearance was observed during binding. The statistically fitted model of the contour plots was then taken through the optimization tool of Minitab 16 software to determine optimal conditions (Table 1).
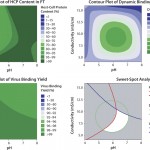
FIGURE 2: Selection of binding conditions of virus on AcroPrep filter plates wIth Mustang Q membrane
Table 1: Data suggest that DBC and recovery are controlled by virus and DNA content in the load. HTS optimization of elution conditions enabled excellent DNA clearance, in addition to the very good HCP clearance already achieved during binding. Note that higher virus recovery is possible but may jeopardize DNA clearance.
Transfer of Selected Conditions Defined by HTS to Acrodisc Units with Mustang Q XT Membrane: The conditions for optimal bind/elute of influenza virus determined on the AcroPrep Advance 96-well filter plate with Mustang Q membrane in the HTS approach were successfully applied on an Acrodisc unit with Mustang Q XT membrane. Data (not presented here) show virus DBC in the upper 1011 range. Elution yielded good recovery of 75 % with excellent and constant DNA and HCP (>95%) clearance and process productivity (viral particles purified per hour). (Data and studies performed are presented in Reference 2 and available at www.pall.com/pdfs/Biopharmaceuticals/MustangQXT_AcroPrep_USD2916.pdf).
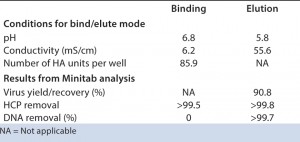
TABLE 1: Selected conditions derived from DoE analysis results (Minitab software) HTS on AcroPrep Advance filter plates with Mustang Q membrane
Conclusions
The prepacked format of Mustang devices reduces the hardware investment associated with chromatography and allows for more flexible operation, including single-use, eliminating the need for cleaning validation. Barriers to implementation of membrane chromatography include a lack of experience with these methods linked to concerns about cost and yield. As with all chromatography, highest yields with best purities result from optimization of the various parameters influencing the quality attributes, but this requires time and resources. The study shows how barriers are overcome with a structured DoE and HTS approach to define the optimal operating conditions.
The conditions for optimal bind/elute of influenza virus on the Mustang Q membrane, determined on the AcroPrep Advance 96-well filter plate in the HTS approach, successfully matched data obtained on Acrodisc unit with Mustang Q XT membrane, confirming the validity of the conditions determined by the HTS approach. In the context of the purification of influenza virus, the study demonstrates the predictive power of this methodology. The ratio between DNA and virus load is important for best recovery and purity. The HTS approach is fast, consumes minimal sample, provides useful information about parameters such as the virus binding capacity, and allows for reliable transfer and scale-up to a chromatography capsule.
It needs to be emphasized that the purification was performed with a virus feedstock that was processed only through a depth filter for cell removal. There was no DNA reduction treatment with endonucleases before the load on the Mustang Q XT membrane. The positioning of the Mustang Q membrane capture/elute step directly post cell removal eliminates the tangential flow filtration step typically combined with endonuclease treatment.
The study confirmed that Mustang Q XT capsule is a valuable alternative for purification of influenza virus, and by extension for other viruses, from clarified cell culture feedstock, allowing for faster, simpler, and economical processing
References
1. Le Ru, A. Scalable Production of Influenza Virus in HEK-293 Cells for Efficient Vaccine Manufacturing Vaccine 2010(28): 3661-3671
2. Purification of Influenza Virus by Ion ExchangeChromatography on Mustang® Q XT Membranes. Fast Purification Conditions Screening on AcroPrep Advance 96-Well Filter Plates Pall Life Sciences Application Note USD2916
Catherine Allioux is global product manager, chromatography sorbents and membranes, 25 Harbor Park Drive, Port Washington, NY 11050; 33-13-420-7824; catherine_allioux@europe.pall.com; www.pall.com.
2013, Pall Corporation. The Pall logo, Acrodisc, AcroPrep, and Mustang are trademarks of Pall Corporation. Filtration.Separation. Solution is a service mark of Pall Corporation. ® indicates a trademark registered in the USA, and ™ indicates a common law trademark.

Hi Catherine – Nice piece!
Hope all goes well. Let’s stay in touch.
Best regards,
Jim