The introduction of recombinant proteins and monoclonal antibody (MAb) products revolutionized the treatment of many diseases, including diabetes, rheumatoid arthritis, multiple sclerosis, Crohn’s disease, cardiac disease, and cancer. These highly specific biologic therapies provide patients with life- saving approaches that are not possible with small molecules. MAbs in particular are a unique class of biopharmaceutical products that interact with and activate components of the immune system to provide such therapeutic benefits as tumor destruction by antibody-dependent cell-mediated cytotoxicity (ADCC). Other biopharmaceutical products such as growth factors can activate receptors and stimulate a cell-based response, and enzymes can provide critical function in target tissues. In addition the unique affinity and specificity of biologic molecules to bind tightly to receptors and cell surface antigens can be exploited to deliver highly potent payloads to target tissues. The recently approved Adcetris drug conjugate, an anti-CD30 MAb attached by a protease-cleavable linker to the microtubule disrupting agent monomethyl auristatin, is an example of this latter class of biopharmaceutical products (1).
With the increasing knowledge of the human genome and the resulting greater depth of understanding of the molecular basis of many diseases, biopharmaceuticals will continue to play a major role in the treatment of human disease worldwide for the foreseeable future. The success of biopharmaceuticals is highlighted by the increasing sales over the past decade. Sales in the United States and Europe have grown steadily, reaching US$107 billion in 2010 — approximately 12% of the total US$856 billion pharmaceutical market in these areas (2,3). Sales of biopharmaceutical products are expected to increase at a compound annual growth rate (CAGR) of >9% through 2015 to an estimated US$167 billion.
Although biopharmaceutical products will continue to play an important role in treating disease, cell therapy — the process of introducing new cells into a tissue to treat a disease — is emerging as a novel and specific means of treating degenerative diseases such as heart failure and arthritis and acute diseases such as stroke or spinal cord injury, which are difficult or impossible to treat with today’s biopharmaceutical products.
Cell therapy is generally the administration of living cells to replace a missing cell type or to provide a continuous source of a necessary factor to achieve a truly meaningful therapeutic outcome. The many potential forms of cell therapy range from transplantation of cells derived from an individual patient (autologous cell therapy) or from another donor (allogeneic cell therapy). Autologous cell therapy historically has been favored because of its lack of required immunologic matching. In allogeneic cell therapy, cells are harvested from one donor or a few universal donors followed by large-scale expansion and banking of multiple doses. The expanded cell preparations are cryopreserved for later manipulation or as therapeutic doses. Allogeneic cell therapy uses cell types that do not elicit immune responses upon implantation and therefore have the potential to treat hundreds of patients from a single manufactured lot of cells.
The allogeneic methodology fits the pharmaceutical model of drug manufacturing because such products can be readily available for “off the shelf ” distribution. The cells typically used in cell therapy, whether autologous or allogeneic, can be either unmodified or genetically engineered. They may contain pluripotent or multipotent stem cells, differentiated cells that have the required phenotype, immune cells that are administered to enhance the native immune response, or other cell types designed to treat the target indication.
Cell therapies are being developed to address a large number of previously unmet medical needs. Although these products hold great promise, there are also many challenges and hurdles to their successful development and widespread use. Uncertainties over how cell therapies should be regulated, safety concerns over administration of live cells to a patient, and lack of sufficient funding to support the full development and commercialization of cell therapies all present significant challenges to the development of cell therapies.
In addition, one of the biggest challenges is in developing reliable and robust manufacturing processes for these products that ensure adequate product safety, potency, and consistency at an economically viable cost. As noted by Alexey Bersenev, cell therapy is very different from all other existing therapies, including biopharmaceuticals, and regulation of both the development and manufacture of cell therapies under current good manufacturing practices (CGMP) statutes is a challenging issue (4). Cell therapy manufacturing processes can range from very short ex vivo processes to a simple expansion of autologous cells for administration back to a patient — to long cycles of culture, expansion, stimulation or treatment with external factors, or genetic manipulation of allogeneic cells that are banked for treating multiple patients. So the regulations of cell therapy manufacturing processes must be broad enough to cover this range of processes while still ensuring the safety and potency of a final product.
Comparisons to MAbs: Although manufacture and regulation of cell therapy products can appear daunting, many issues and concerns being raised about these products and their manufacturing processes are similar to those expressed when recombinant proteins and MAbs were initially developed. In the United States, most recombinant proteins and MAbs were originally regulated under the Public Health Act similarly to vaccines and biologic products derived from human plasma. Because of the complexity of these products and difficulties in completely characterizing them and their manufacturing processes, the concept that “the process defines the product” resulted in stringent regulations regarding manufacture of biopharmaceuticals. The products were defined by their manufacturing processes and the facility in which they were manufactured (including scale and specific equipment used in processing operation).
Beginning in the mid-1990s with the FDA’s publication of guidances defining well-characterized biologics and use of comparability protocols to support process changes during development and after product approval, more flexible and responsive biopharmaceutical manufacturing regulations began to emerge (5). Those have allowed the biopharmaceutical industry to flourish. Our review of the history of MAb product development and manufacturing highlights advances that have been made in the technical and regulatory aspects of biopharmaceutical development. Many of the lessons learned during the past 20 years of MAb development can be applied to today’s cell therapy products and their manufacturing processes. We anticipate that these lessons will facilitate the maturation of cell therapy manufacturing processes and help accelerate bringing these exciting new products to market.
Th
e first recombinant protein biopharmaceutical products were predominantly low-dose hormones and growth-factor products requiring relatively low volumes of manufacturing capacity with minimal challenges in achieving economic viability. When they were first introduced, therapeutic MAb products represented new manufacturing challenges due to the relatively high doses that were required to obtain a beneficial therapeutic outcome and the relatively low yields observed in their manufacture. With doses ≤ 5 mg/kg, the challenge of reliably and economically producing such large quantities of product delayed the transition from the initial discovery of MAbs to the licensing and distribution of the first successful products for more than a decade. The transition of cell-based therapies from early development to full commercial success is likely to take a similar period of time and require the coordinated efforts of scientists and regulators to develop a complete understanding of the critical quality attributes of cell therapies and the impact of different process conditions on those attributes.
Brief History of MAbs As Commercial Biopharmaceuticals
In 1984, Kohler and Milstein received the Nobel Prize in Medicine for their pioneering work on the production of MAbs (6). Creation of an immortalized cell line as a continuous source of a single antibody revolutionized the use of these products in medicine and enabled their development as therapies for human disease. By 1986, Orthoclone OKT3 (Ortho Pharmaceuticals), the first MAb for human use, was approved for prevention of kidney transplant rejection. The enthusiasm for therapeutic MAbs continued through the 1990s with the next wave of antibody products generally being developed as anticancer agents. MAb products continued to gain marketing approval through the decade. Many of the products approved in the 1990s are among the top 10 biopharmaceuticals on the market today.
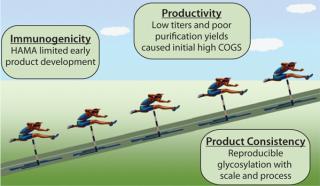
Challenges in Early Antibody Development: Although MAbs are now a well-established product class, reaching the current status quo was not a smooth path. As illustrated in Figure 1, issues such as the immunogenicity of the earliest antibody products, low productivity of manufacturing technologies, and challenges in making a consistent product with the inherent variability of mammalian cell-based expression systems all contributed to delays and set-backs as these products were developed. It is safe to say now, however, that the industry focused on solving theose problems and developing MAbs into the generally safe and effective therapies that they are today. Cell therapy will face similar hurdles, but with the necessary attention and resources industry will solve those problems and advance new therapies into the market to treat currently untreatable diseases.
Addressing Manufacturing Challenges: Biopharmaceutical products must be manufactured cost-effectively to meet the market demands and to achieve commercial success. This was also a major challenge for early MAb producers. The relatively high doses of these products forced the industry to focus on improving productivity and efficiency of manufacturing processes. The focus was particularly on increasing the protein production level (titer) achievable in the cell-culture bioreactor and the overall purification yield in downstream processing. Early on, expression levels in cell culture for MAbs were typically on the order of 100–500 mg/L. Even as recently as five years ago, antibody titers in excess of 1 g/L were uncommon, and many products were launched using production cell lines and manufacturing processes with bioreactor titers of 0.5– 1.0 g/L (7). Industrial and societal pressure to produce more product at reasonable costs pushed process scientists and engineers to develop methods for generating high-expressing production cell lines and culturing these cell lines for maximum productivity. Optimization of cell lines and fed-batch processes over the past 20 years led to steadily improving antibody titers. Today, titers of 5 g/L or more are becoming routine, and the cost of goods for future products is therefore decreasing (8).
A significant contribution to improving antibody manufacturing processes was the establishment of the Chinese hamster ovary (CHO) cell as the platform production host cell line for antibody production. Establishment of a platform cell line and process across the industry has reduced technical and regulatory risk for new product development and has reduced the commercial cost of goods for products that use the new production technologies. Applying the same principles to cell therapy may not be possible, but industry should focus on building platforms, media, process understanding, and overall manufacturing paradigms that are easily replicated across multiple types of cell sources and therapeutic modalities. Approaches to achieve process platforms and operational excellence in cell therapy manufacturing are just beginning to be implemented. These should lead to more cost-effective cell therapy products in the future.
Unique Cell Therapy Challenges
As noted above, cell therapy can be either autologous or allogeneic. Both types of therapies are more challenging than MAbs to produce safely and consistently, but from a manufacturing viewpoint, autologous cell therapy presents a greater set of challenges. Each such dose is a single batch and is derived from a different source. Although the cell type will be the same for a given therapeutic approach, the cells from different patients will still behave differently when cultured. Source material will contain a variety of cell types with varying growth and differentiation capacity. The capacity of cell therapy products to differentiate in vivo into the desired cell type and to exhibit the intended mode of action will strongly depend on the conditions and time of in vitro culture, such as the use of growth factors and the choice of separation methods.
With autologous therapy, processing multiple small batches results in little, if any, economies of scale, which greatly increases production costs. Scale-up of autologous cell therapy manufacturing requires not the use of larger processing equipment, but rather, the increased handling of small, laboratory-scale equipment. The challenges of this “scale-out” are significant and require the extensive use of automation to handle a large volume of batches and keep track of the myriad operations required to reproducibly process each patient’s cells without cross-contamination or mix-ups. Furthermore, because each patient’s cells must be processed and tested separately, the level of documentation required is high. Testing represents a significant portion of the final cost per patient.
As a result, many companies developing autologous cell therapies envision using multiple manufacturing sites and processing centers to distribute the workload and minimize the shipping distances for such time- sensitive products. The Provenge prostate-cancer therapy from Dendreon was approved by the FDA in 2010. To meet demand, that company is now progressing down the route of increasing manufacturing capacity production scale-out.
Unlike autologous cell therapies, allogeneic produ
cts are often manufactured centrally and are generally capable of “scale-up” to accommodate larger batch sizes. One principal challenge of an allogeneic therapy is maintaining product and process consistency through the different stages of development, including production at different manufacturing sites or at different scales. Automated procedures are also being evaluated by companies developing allogeneic products to reduce errors, improve process consistency, and help lower costs. This is similar to the drive to increase production titers for antibody products to make them more cost-effective to manufacture. Cell therapy companies can look toward the steps that antibody companies took to optimize processes and reduce manufacturing costs for guidance on methodical approaches to process optimization — albeit with the added challenges that cell therapies pose in terms of product-quality considerations.
Similar to MAb manufacturing, cell therapy companies must apply as many in-process and final-product release methods as possible to know that the process can produce a cell therapy product with the desired critical quality attributes. Many cell therapy products are fragile preparations that must be shipped and applied to a patient rapidly. This time pressure means that standard product-release testing procedures are not feasible. In particular, sterility testing often cannot be completed before patient treatment. This unique challenge in cell-therapy manufacturing requires tighter environmental and handling controls to greatly reduce any risk of sterility failure. These tight, batch-specific controls go beyond those applied to MAb testing.
In addition to process consistency and product sterility, cell-derived products pose unique risk factors. An important safety concern is the capability of (in particular) embryonic stem cells and induced pluripotent stem cells to form teratomas (germ-cell tumors comprising multiple cell types). Although benign, their formation in anatomically sensitive positions, such as the central nervous system, is an important safety concern, and the risk of ectopic engraftment in nontarget tissues must be addressed. It is also acknowledged that additional appropriate structural and morphological endpoints may be necessary to study regeneration, repair, or replacement of a tissue (9).
Continual Improvements
Advances in cell-line generation and cell culture described above for antibodies have enabled the large-scale commercial manufacturing of MAbs at reasonable costs. As a result, early concerns that the industry would be unable to meet the growing demands for production of MAbs have subsided. Today, companies are continuing to improve antibody manufacturing processes through implementation of new technical and regulatory initiative such as process analytical technology (PAT), quality by design (QbD), and other new regulatory concepts to further reduce cost and development timelines for MAbs without adversely lessening their quality.
Cell therapy and other regenerative medicine products now in development are at the cutting edge of a new therapeutic paradigm whose applications across a broad spectrum of disease can be anticipated if these therapies prove to be as effective as initial reports suggest. The industry should focus on implementing the relevant principles of PAT and QbD that are directed toward detailed process and product understanding, knowledge of raw materials and process interactions, and definition of operational design space. Further, development of rapid, sensitive in-process and release-testing approaches for cell therapy will enable these unique products to be produced, tested, transported, and administered cost-effectively and safely. Lessons from the early days of MAb development can help guide the efforts in this new therapeutic modality.
Author Details
Corresponding author Susan Dana Jones is vice president and senior consultant, and Howard L. Levine is founder, president, and principal consultant at BioProcess Technology Consultants, Inc., 12 Gill Street Suite 5450, Woburn, MA 01801 USA; sjones@bptc.com. Susan McKee is business development manager at Angel Biotechnology PLC, Pentlands Science Park, Nr Edinburgh, Scotland, UK EH26 0PZ.
REFERENCES