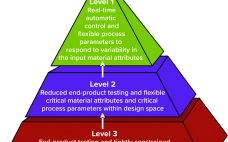
Process control enables biomanufacturers to ensure that operating parameters are within defined specifications. A control strategy should be established during early stages of process development while process and product performance are being defined using risk-based methods such as quality by design (QbD) and process analytical technologies (PATs). Confirming process control as an essential part of product development creates greater process knowledge and understanding and provides the first steps toward process optimization. By understanding how process performance relates to product quality,…
Friday, May 15, 2020 Daily Archives
Swiss biomanufacturing revived in 2019 says industry group
Swiss biomanufacturing had a “resurgence” in 2019 according to an industry group which cited plant investments by drug firms and CDMOs as growth drivers. The Swiss Biotech Association – which represents biopharmaceutical companies, biotech and the services sector – made the comments in a recently published report. It wrote that, “Switzerland’s manufacturing prowess has experienced a revival in 2019, following years of “exporting” manufacturing capabilities aboard.” The organization highlighted Biogen’s decision to invest CHF 1 billion ($1 billion) in a…
Takeda warns of failures and bottlenecks in COVID vaccine rush
Takeda says it is looking to partner to enter the COVID-19 vaccine race but warns industry of the difficulties of developing and manufacturing such products. Industry’s response to the coronavirus pandemic has been unprecedented. According to data provided by Pharma Intelligence, there are – as of Friday 15 May – 116 vaccines in development against COVID-19, nine of which are being trialed in humans. But while pharma giant Takeda has welcomed the surge in COVID-19 programs, CEO Christophe Weber has…
FDA demands more manufacturing info to review ide-cel, says Bluebird
The US FDA has rejected Bluebird Bio’s Biologics license application (BLA) for ide-cel and asked for more details of how the candidate blood cancer therapy is made. Bluebird announced receipt of a “refuse to file letter” (RTF) in an SEC filing on Wednesday. The firm said the US Food and Drug Administration (FDA) asked for supplemental information on validation and control processes used in lentiviral vector and drug product manufacturing processes for Idecabtagene vicleucel (ide-cel), an investigational chimeric antigen receptor…