Careful consideration of lot size is crucial for multiyear success of a cell therapy business. RoosterBio’s product design and acceleration business works with the company’s customers to help make plans that are appropriate for their stages of product and clinical development. We use the following major considerations in creating a sound multiyear strategy with intermediate milestones: Understand what the future looks like and work backward Use reasonable to conservative assumptions to estimate a range of needs Invest in the right…
Monday, October 21, 2019 Daily Archives
Rapid Mammalian Cell Harvest Without Centrifugation for Antibody Purification: Using a Novel System for Cell Culture Media Clarification
Monoclonal antibody (MAb) expression systems typically use signal peptides to ensure secretion of antibodies into cell culture media. Although that reduces the complexity of purification and prevents the need for cell disruption, it does require using expensive and time-consuming techniques to separate cells from antibody-containing cell culture fluids. In this study, we describe our tests of the novel Sartoclear Dynamics Lab V system (Sartorius S Lab Instruments GmbH and Co. KG) for rapid clarification of cell culture media without requiring…
Special Report: Current Analytical Approaches to Biophysical Characterization in a Regulatory Environment
Structural integrity of protein-based therapeutics is one of the major challenges in the biopharmaceutical industry. Multiple factors such as product stability, efficacy, and shelf life could be affected following minor changes in manufacturing process. Multiple biophysical methods employing spectroscopic and calorimetric tools can be used to analyse Higher Order Structure (HOS). Moreover, with an increasing demand for generating as much structural information as possible for regulatory submissions, a requirement for these analyses in a GMP environment is also important. This…
Technology Highlights from the 2019 BioProcess International Conference
This year’s BioProcess International Conference and Exhibition, held 9–12 September 2019, hosted nearly 200 exhibitors showcasing technical innovations for the biopharmaceutical industry. This year’s conference sessions also included daily technical workshops detailing supplier solutions and technologies. Below are some notable technologies featured that demonstrate the industry’s dedication to finding new ways to help manufacturers shorten time to market. Most systems offer the benefits of single use, integration, process control, economies of scale, automation, and closed-system processing. Upstream Production The exhibit…
Smart Sensors and Data Management Solutions for Modern Facilities
Bioprocess manufacturers continue to seek technologies for increasing productivity and shortening timelines from discovery to commercialization. Innovations such as high-throughput systems, automated platforms, and the latest clarification systems all have made processes efficient and robust. And with the increasing adoption of quality by design (QbD) principles, including the use of process analytical technologies (PAT), biomanufacturers are mitigating the risks of errors in their operations better than ever before. A critical part of mitigating risk is gathering meaningful process data and…
Rapid Implementation of Novel Affinity Purification: Manufacture of Commercial-Scale Next-Generation Antibody Therapies
The rapid and cost-effective production of conventional monoclonal antibodies (MAbs) for clinical trials and commercial supply has contributed toward their wide adoption. Production processes have become more efficient because common purification processes are being used across structurally similar MAbs during key steps of process development and manufacturing. Such successful platform approaches can remove unwanted impurities and are stable across processing conditions, irrespective of the MAb being purified. In addition, they are readily available at the required volume to support large-scale…
CMC Development Platforms and Outsourcing to Reduce Timelines
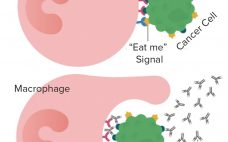
Forty Seven is a company developing novel therapies based on anti-CD47 and other immuno-oncologies. CD47 is called the “don’t eat me” signal that cancer cells give out to escape elimination by the body’s immune system. Qinghai Zhao, vice president of technical development and manufacturing, is one of the scientists working on the company’s magrolimab (5F9) monoclonal antibody that is designed to block the binding of the CD47 signal to the cell receptor SIRP-α while boosting the “eat me” signal that…
Danaher offloads assets to Sartorius for $750m; GE buy pushed back to 2020
Sartorius will add biomolecular characterization, chromatography hardware and resins, and microcarriers in the deal, dependent on the closure of Danaher’s acquisition of GE Healthcare’s Biopharma business. Earlier this year, Danaher Corporation announced its intention to increase its presence in the bioprocessing space through the full biopharma portion of GE Healthcare’s Life Sciences business for approximately $21.4 billion (€19.2 billion). The GE business unit will, once the deal closes, become the latest subsidiary of Danaher, which boasts Pall Corporation, Sciex, and…