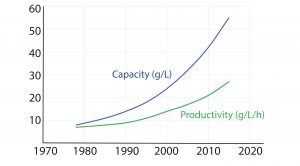
Figure 1: Protein A chromatography resin capacity and productivity (adapted from Bolton G, et al. The Role of More Than 40 Years of Improvement in Protein A Chromatography
in the Growth of the Therapeutic Antibody Industry. Biotechnol. Prog. 32, 2016: 1193–1202)
The monoclonal antibody (MAb) market has grown over the past decade to be about half of the biomanufacturing market today. This growth should continue, driven by a strong pipeline of MAbs that are currently in phase 2 and 3 clinical trials. A synergistic evolution of MAbs and protein A resins also has taken place in the market. Figure 1 shows a graph adapted from an Amgen study of the development of protein A productivity and capacity over the past 40 years. Protein A has made MAbs highly manufacturable and easier to develop in laboratories. The purification platform approach, which has been enabled by protein A, has been a key element in this market growth.
As Figure 1 shows, productivity of protein A resins has increased >4% annually since 1978 and capacity about 6% annually. Despite that, opportunities exist for improving protein A technology. Upstream titers have improved dramatically, increasing nearly 100-fold over the past four decades. As such, protein A has become the rate-limiting step in some downstream purification suites.
In general, protein A columns are too large, which means that they either limit the use of prepacked technology, or they create a mismatch between a protein A column and downstream operations. Protein A columns also are more prone to bioburden contamination than any other step in a downstream process. Protein A resin is a reused raw material. It’s exposed to the highest load of nutrients as the entire harvest from the bioreactor is loaded onto the protein A column, but it is cleaned with weaker clean-in-place (CIP) chemicals than other downstream steps. Typically, ion-exchange and hydrophobic interaction chromatography (HIC) resins are cleaned with 1.0 M sodium hydroxide. But in a vast majority of cases today, protein A resin is cleaned with 0.1 M sodium hydroxide. At the same time, regulatory agencies are increasingly interested in sources of bioburden and the methods that manufacturers are using to limit the outbreak of bioburden in their manufacturing areas. And despite generational improvements that have led to lower cost of ownership for protein A, further cost reductions are needed.
Please log in to access this content.
Learn more:

Jonathan Royce is Global Business Leader for Chromatography Resins at GE Healthcare Life Sciences.
