Protein A has made monoclonal antibodies (MAbs) highly manufacturable and easier to develop in laboratories. Its use has enabled purification platform approaches, which have been key elements in growth of the MAb market. But in general, protein A columns are too large, which means they either limit the use of prepacked technology, or create a mismatch between a protein A column and downstream operations. Protein A columns also are more prone to bioburden contamination than any other step in a…
January 2018 GE Special Report
Addressing the Risk of Bioburden and the Need for Increased Productivity in Protein A Chromatography
Protein A has been a fantastic tool for the antibody industry. It is without doubt the most well-established purification technique in monoclonal antibody (MAb) manufacturing today due to a few key success factors. Protein A is the result of the long evolution of Staphylococcus aureus that developed a defense system against antibodies. Protein A exists on the cell wall of about 9% of S. aureus strains and immobilizes IgGs. When there is an immune response in the body, the bacterium…
Evaluation of a Next-Generation Protein A Chromatography Resin for the Purification of Monoclonal Antibodies
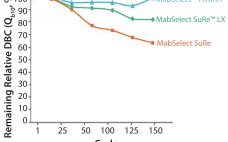
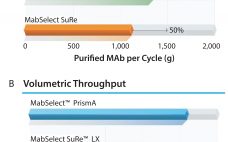
Next-generation, high-capacity, alkali-stable protein A resins have recently become available in anticipation of increased production and throughput demand for protein therapeutic processes. Efficient caustic cleaning and bioburden control regimens have allowed agarose-based, alkali-stable protein A chromatography resins to become the backbone of many commercial purification processes for over a decade and have served as the primary capture step for monoclonal antibody (MAb) purification processes. However, the relatively high raw material costs, along with limitations to binding capacity and aggressive resin…
Reimagining Capacity for Today’s Purification of Monoclonal Antibodies
The monoclonal antibody (MAb) market has grown over the past decade to be about half of the biomanufacturing market today. This growth should continue, driven by a strong pipeline of MAbs that are currently in phase 2 and 3 clinical trials. A synergistic evolution of MAbs and protein A resins also has taken place in the market. Figure 1 shows a graph adapted from an Amgen study of the development of protein A productivity and capacity over the past 40…
Thirty Years of Monoclonal Antibodies and Protein A: A Retrospective
In 1980 at the University of Cambridge’s department of pathology, I worked with Herman Waldmann to develop monoclonal antibodies (MAbs) as treatments for graft-versus-host disease (GVHD). That disease is associated with serious complications of stem cell transplantation when attacking T-cells can damage the lungs, liver, skin, and other organs. If we could find a specific MAb that would work with the human complement system to kill those cells, they could be selectively removed from the bone marrow. The human complement…
A Discussion Regarding the Productivity in Downstream Operations
At the Biotech Week Boston conference (24–28 September 2017), BioProcess International publisher Brian Caine had the opportunity to speak with Jonathan Royce, global business leader for chromatography resins at GE Healthcare. Their discussion covered current downstream challenges and the company’s next-generation chromatography resin. Industry Challenges Caine: What are the most critical purification challenges for biomanufacturers? Royce: The bioindustry has seen more than a 100-fold increase in the productivity of cells. This means that downstream purification technology must continue to evolve…