Next-generation, high-capacity, alkali-stable protein A resins have recently become available in anticipation of increased production and throughput demand for protein therapeutic processes. Efficient caustic cleaning and bioburden control regimens have allowed agarose-based, alkali-stable protein A chromatography resins to become the backbone of many commercial purification processes for over a decade and have served as the primary capture step for monoclonal antibody (MAb) purification processes. However, the relatively high raw material costs, along with limitations to binding capacity and aggressive resin stripping strategies with current resins, still represent a high contribution to overall manufacturing cost of goods. To address this, a novel higher capacity prototype protein A resin from GE Healthcare (MabSelect™ PrismA) was evaluated using a CHO-expressed MAb. Stability and performance of the resin over its usable lifetime was studied by monitoring dynamic binding capacity (DBC), protein A ligand leachate, and total protein carryover.
Protein A is a 42 kDa protein found on the cell wall of Staphylococcus aureus. Staphylococcal protein A (SpA) is composed of five IgG-binding domains (E, D, A, B, and C) folded into a triple helix bundle. All domains have affinity for, and can specifically bind to the Fc region of antibodies. It is also known that multiple domains have affinity for the antigen-binding region (Fab) of the antibody, most notably the D domain (VH3 family). Many companies have purposely modified and mutated these domains to ensure high stability, specificity, and consistency of affinity purifications over the lifetime of the resin. These modified domains, due to their increased stability, have been produced as repeating subunits (repeating tetramer, pentamer, or hexamer) to further increase efficiency in binding capacity.
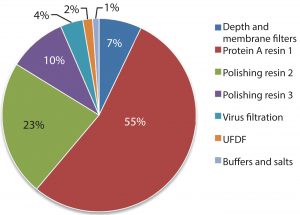
Protein A resin accounts for a large portion of the overall raw material costs to MAb processes, (Figure 1) and to a significant portion of overall operating costs (1) and cost of goods manufactured (COGM) (2). To drive down total COGM, either the raw material price must be lower or higher raw material utilization must be employed.
Please log in to access this content.
Learn more:

References
1 Tosoh BioScience Application Note A1528A: Protein A Chromatography — The Process Economics Driver in MAb Manufacturing.
2 Kobayashi S, Ueda Y. Comparing Protein A Resins for Monoclonal Antibody Purification. BioPharm Int. 26(12) 2013.
3 Kaltenbrunner O, et al. Continuous Bind-and-Elute Protein A Capture Chromatography: Optimization Under Process Scale Column Constraints and Comparison to Batch Operation. Biotechnol. Progress 32(4) 2016: 938–948; doi:10.1002/btpr.2291.
Bryan Dransart is a Senior Research Scientist I at Gilead Sciences. April Wheeler was formerly an Associate Scientist at Gilead Sciences (currently Technology Manager at MilliporeSigma). Tony Hong was formerly a Rearch Scientist 1 at Gilead (now retired). Chi Tran is Senior Research Associate II. Rafael Abalos is a Senior Research Associate II; Andrew Quezada is a Senior Research Associate I; ShiYu Wang is a Senior Research Associate I; Brian Kluck is a Research Scientist 1; and Nooshafarin Sanaie is a Senior Research Scientist II, all at Gilead Sciences.