So many manuscripts we receive begin by mentioning the “ever-growing” or “ever-expanding world of biopharmaceuticals.” For anyone who’s been covering this industry for a while, that’s an understandable impression — but it’s not always easy to express. Despite increasing in maturity, that “world” remains on the cutting edge of science and technologies for innovative therapeutic development, and its entrepreneurial spirit remains strong. But we’re all accessing information in different ways now than in the past. We need and want that…
Friday, June 1, 2018 Daily Archives
May Spotlight
Welcome New BPI Colleague In April, BioProcess International waved a reluctant goodbye to European strategic marketing consultant Joanna Hendrikx. Pregnant with her second child, she is looking forward to devoting herself exclusively to motherhood. We will miss her energy, finesse, and sense of humor even as we welcome our new colleague taking over her role here. Victoria Biscoe has a long history with Informa, our parent company, having joined in 2010 as a key account manager for the Informa Life…
A Product–Packaging Interaction Study to Support Drug Product Development
Drug packaging is subject to a number of regulatory requirements, including those for product containers and packaging. For example, according to the federal Food, Drug, and Cosmetic Act (FD&C) section 501(a)(3), a drug is considered adulterated “if its container is composed, in whole or in part, of any poisonous or deleterious substance which may render the contents injurious to health.” And 21 CFR states that drug packaging “shall not be reactive, additive, or absorptive so as to alter the safety,…
Host-Cell Protein Risk Management and Control During Bioprocess Development: A Consolidated Biotech Industry Review, Part 1
Host-cell proteins (HCPs) constitute a significant class of process-related impurities during biologics manufacturing. Due to their potential impact on product quality and efficacy as well as patient safety, the total amount of residual HCP in a biological drug substance generally is considered a critical quality attribute (CQA) that usually needs to be tested for during batch release (1, 2). It is both an “industrywide” common understanding and a regulatory requirement to remove HCPs from biologics to acceptably low levels that…
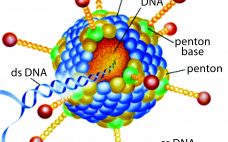
Development of a Freeze-Dried Ebola-Expressing Adenoviral Vector: Unexpected Findings and Problems Solved
In December 2013, a two-year-old child in Guinea became the first person to be killed by Ebola in the most recent outbreak. In March of the following year, that outbreak was declared in West Africa. By mid-2014, the World Health Organization (WHO) had declared it to be a public health emergency of international concern and urged pharmaceutical companies to accelerate their development of candidate vaccines. At the peak of the outbreak in 2014, more than 1,200 new cases of Ebola…
Rational Design of Liquid Protein Formulations: Application of Biophysical Stability Predictors and Descriptors to Reformulate Biotherapeutics
Successful development of liquid biopharmaceutical formulations requires careful assessment of the biophysical properties of the protein in solution, primarily focused on achieving optimal conformational and colloidal stability of the drug-substance molecule (1–11). It also involves extensive stability studies under stressed conditions. Using state-of-the-art biophysical tools for characterization of developed products, those studies are based on key biophysical descriptors and extended particulate characterization methods (subvisible particles in micro- and nano-size range) to deliver a stable product for market with a shelf…
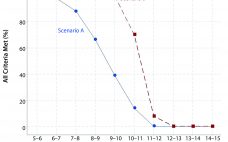
Statistical Assessments of Bioassay Validation Acceptance Criteria
Analytical linearity as well as assessments of precision and accuracy determine the range for a bioassay (1). USP <1033> recommends comparing confidence intervals (CIs) against target validation acceptance criteria in a bioassay validation exercise, but there are no clear guidelines for determining the criteria (2). Here I address several aspects of a bioassay validation, namely • Linearity (coefficient of determination (R2), slope, and intercept parameters) • Accuracy (%relative bias, %RB) • Precision (percent coefficient of variation, %CV) CIs for the…
Large-Volume Wearable Injectors: A New Delivery Approach Could Change the Game
Biologic drugs are a driving force in the pharmaceutical industry. More than 2,700 were in pharmaceutical pipelines as of June 2017 to treat cancers (836), rare diseases (566), neurologic conditions (420), autoimmune disorders (311), and other ailments (567) — triple the number in 2013 overall (1). Biopharmaceuticals make up more than half of all drugs in development and are nudging out traditional small-molecule drugs rapidly while already bringing in billions of dollars in sales. The success of such drugs is…