For current process development phases, many biomanufacturers’ attention is directed increasingly to the first unit operation in downstream processing, which is the removal of cells and cell debris from culture broth and clarification of supernatant containing a biopharmaceutical product. Given the high cell densities achievable with both mammalian and microbial cell culture processes, primary recovery can be a significant challenge.
The current trend in cell culture is to increase product titers with enriched culture media, improved cell productivity, and increased cell mass. High titers also can be achieved through increased culture duration, which can lead to a significant drop in cell viability. These factors cause the increase of process impurities such as host-cell proteins (HCPs), nucleic acids, lipids, and colloids, as well as generation of a broad particle-size distribution in cell culture fluids. Downstream chromatography requires a fast and reliably produced particle-free supernatant.
Centrifugation
Centrifuges perform a robust clarification process. This is a common technique used to harvest large-scale cell culture vessels because it combines low running costs with uncomplicated process development and operating robustness (2–4). Multichamber, tubular bowl, and disc-stack designs all are available. Centrifuges remove a considerable proportion of cells and cell debris that can foul downstream filters and chromatographic steps, leading to unacceptable pressure drops and reduction in overall performance. An optimized centrifugation process minimizes cell lysis — and related generation of additional cell debris or release of intracellular impurities and proteases — and maximizes sedimentation of submicron particles and product yield (5).
Most current harvest operations make use of hermetically sealed, bottom-fed centrifuges that completely eliminate the air–liquid interface commonly present in standard nonhermetic centrifuges. That interface can be harmful to the integrity of the mammalian cells, and it creates additional cell debris when not managed appropriately through backpressure. Fully hermetic centrifuges use mechanical seals that isolate product fluid from outside air, eliminating cell-damaging air–liquid interfaces by enabling the machine to be completely filled with fluid. Such a design also eliminates valves and pumps from the inlet piping, which contributes to an overall reduction in cell lysis by >50% (6, 7).
Single-use options include CARR Centritech’s UniFuge system from PneumaticScaleAngelus. Its gamma irradiated, disposable module eliminates the need for cleaning in place (CIP) and steaming in place (SIP). The unit provides relatively low shear forces that will not compromise the integrity of animal cells. However, a limited feed flow rate of ≤6 L/min constrains this centrifuge to batch volumes of 2,000 L or less, with cell culture harvesting periods of six hours at minimum.
Alternatively, kSep single-use centrifuges from Sartorius Stedim Biotech can handle up to 6,000-L culture volumes with a throughput of 12 L/min. Both systems can be applied for continuous processing, retaining cells in a bioreactor while harvesting culture supernatant. Scale-down models for centrifugation are not always representative, however, which complicates linear scale-up in process development. In addition, centrifugation cannot completely remove the submicron-particle load from a product stream. Depth filtration remains a mandatory step before loading the first chromatography step downstream.
Tangential-Flow Microfiltration
Flow filtration is the simplest procedure for clarifying cell culture supernatants of nearly unlimited scale. Tangential-flow filtration (TFF) is often used based on its ability to counteract continually the formation of a surface layer (preventing associated membrane blockage). A cell suspension is pumped through membrane-bounded channels, and flow is adjusted to operate under quasilaminar conditions.
Most companies use TFF cassette systems with open-channel configuration rather than turbulence-promoting installations or hollow-fiber modules. However, significant shear forces are generated along a TFF membrane to prevent filter blockage. Inevitably those are combined with pressure drops in the flow channel, causing transmembrane pressure variation along the channel length and thus a nonhomogeneous flow-through. Filtrate flow and convective transport of particles to the membrane are significantly higher in regions of high transmembrane pressure than in regions of a low transmembrane pressure. This leads to formation of a surface layer and fouling effects, which increase along the length of the membrane channels during filtration. Thus, the available membrane area is reduced continuously, with increasing time of operation. Shear forces cannot be increased further without negative effects on cell integrity.
Single-use TFF options for application in the biopharmaceutical industry include the Allegro CS system from Pall Life Sciences. It can support cell culture supernatants of ≤2,000-L batch volumes with a maximum cassette surface area of 10 m2. Companies maximizing cell culture titers through combined increases in cell density, specific productivity, and culture duration have seen increases in impurity levels and the percentage of solids present in their cell culture fluids. Such increases in submicron debris load made implementation of TFF more challenging, leading some companies to choose centrifugation using heat-sterilizable stainless steel centrifuges coupled with depth filtration. That is the current industry standard for harvesting cell culture fluids from larger — ranging about 5,000–10,000 L — bioreactor volumes.
One TFF variant is Alternating Tangential Flow (ATF) technology from Repligen, which is used primarily for cell retention in perfusion processes. It prevents fouling during long-term applications by creating an alternating flow through the action of a diaphragm, which prevents clogging of the system’s hollow fibers. Recently a presterilized version became available: the XCell ATF6 system, which handles volumes ≤125 L.
Depth Filtration
Cell culture supernatants can be clarified comparatively easily by common-flow (“normal-flow”) filtration. This uses either a membrane with a defined pore size (for dead-end filtration) or a porous material with decreasing pore size along a filter’s thickness (for depth filtration). By contrast with dead-end filtration, in which material is retained on the filter surface, depth filters do not form a filter cake. Additionally, depth filtration can retain particles that are smaller than its pores through a pore-size gradient that separates a broad range of particle sizes (8). Filtration rates differ and are defined by depth-filter manufacturers because no validated standard test method is available. The test for sterile filters, for example, measures their retention of 107 colony forming units (CFU) per milliliter (mL) of the test bacterium Brevundimonas diminuta. When all pores of a depth filter are occupied, a depth filtration process transitions to a dead-end filtration operation.
Depth filters come in several different forms. A common design consists of a layer of cellulose, a porous filter aid such as diatomaceous earth (DE), and a positively charged polymeric resin that binds the two together. Based on those major components, depth filters remove impurities and particulate material through size exclusion as well as adsorption and provide essential protection for membrane filters downstream. For laboratory, pilot, and small-scale clinical applications, users typically place two types of depth filters (a coarse one followed by a tighter one) in series and load them directly with whole cell broth with no centrifugation or TFF step upstream. For larger-scale clinical and commercial operations (>2,000 L), depth filtration is usually coupled with centrifugation.
Depth filters rely on the high porosity of diatomaceous earth (DE). Particles are withheld inside through both sieving and adsorption effects. Typically 10–20 µm in diameter, animal cells are retained mainly through sieving effects; cell debris and small particles need both sieving and adsorption effects for efficient filtration. The adsorption effect also is responsible for binding deoxyribonucleic acid (DNA) and HCPs, but it can bind a protein of interest as well (9).
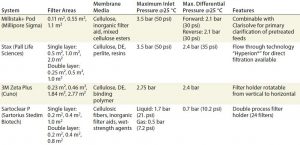
Table 1: Overview of process-scale depth filtration systems (according to manufacturers’ specifications); DE = diatomaceous earth
Several depth filtration systems are on the market (10). All process-scale models — e.g., Millistak+ Pod from Millipore Sigma, Stax from Pall Corporation, 3M Zeta Plus from Cuno Inc., and Sartoclear P from Sartorius Stedim Biotech — can separate cells and prepare culture fluid for downstream chromatographies (Table 1). Equivalent small-scale filters also are available for process development and down-scaled models. Area sizes range from 23 cm2 to 3.7 m2, allowing for free and suitable composition and adaptation to specific volumes and requirements. Process engineers extrapolate the optimal filter area at laboratory scale to a corresponding large-scale filter size. Beyond certain high volumes and particle contents, suitability of depth filtration is limited, but that can be overcome (for example) by sedimentation of culture broth before filtration.
Most depth filters vary by manufacturer in their membrane composition, which is basically cellulose fibers comprising several thousand glucose units that create a fiber labyrinth for retaining particles. Moreover, all manufacturers use filter layers containing DE, which consists of silicon dioxide from the shells of ancient diatoms. DE is characterized by a very porous structure and acid resistance for a high filtration performance. Membranes in 3M Zeta Plus filters additionally contain perlite, which is a volcanic amorphous glass made from crystalline silica (obsidian). Stax filters are made of resins, and Sartoclear P filters contain crosslinking material as a further component.
Process filter cartridges are inserted into a pilot- or a process-scale holder that accepts up to 10 filters per rack. Up to three racks can be used simultaneously for adaptation to any desired scale. These large process filters are not presterilized and must be assembled according to specific process requirements. Filter capsules can be sterilized at 121 °C with 1 bar overpressure for 30 minutes (3M Zeta Plus) or an hour (Stax, Millistak+, Sartoclear P), and they are resistant to sanitization with 1 mol/L NaOH for an hour. Maximum overpressure for operation ranges 1.0–3.5 bar.
Commercially available depth filters can be adapted to any production volume. Encapsulated depth filters from the 3M Zeta Plus EXP SP series have different pore sizes and filter layers. Some units (e.g., 10SP02A, 30SP02A) are suitable for cell separation and protecting chromatographic columns from particles; other filters (e.g., 60ZA05A, 90ZA05A) contain a strong positively charged crosslinking polymer suitable for separating DNA and HCPs. The largest process depth filtration holder allows for a maximum effective filter area (EFA) of 11.2 m2 for a single layer (SL) and 17.5 m2 for a double layer (DL) to handle cell culture volumes ≤5,000 L. Downstream process engineers can use a multiround holder with several racks in a carousel to clarify nearly any production volume efficiently.
Stax filters differ in the content of two different resins. Wet-strength resins increase membrane stability for higher compressive strength. Charged resins induce a zeta potential within the filter, depending on the culture medium used, which promotes separation of DNA and HCPs. These filters come in single- and double-layer forms. The first (coarser) layer is identical in all DL filters. The second (finer) layers can have different pore sizes. The largest Stax SL filter (EFA 2.0 m2) can clarify cell culture volumes of ≤20,000 L.
Millistak+ Pod filters incorporate multiple graded-density layers and adsorptive, positively charged filter media. The D0HC DL model (EFA 1.4 m2) is used for cell separation in largescale processes. Single-use process filters in the Sartoclear P series come as DL filters for clarifying cell culture supernatants. Large-scale process filter systems of all manufacturers are stackable for use in stainless steel housings.
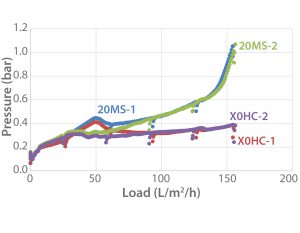
Figure 1: Depth filtration was performed on a MAb-producing recombinant CHO cell (20-µm diameter) suspension containing fed-batch culture supernatant from a 10-L Xcellerex XDR-10 single-use bioreactor (GE Healthcare) and a 10-L BIOSTAT B glass bioreactor (Sartorius Stedim Biotech), each with 7.5-L working volume. Final cell concentration was 1.16 × 107 mL–1, and cell viability was 78%. Total volumes of 4,167 (1) and 4,186 L (2), respectively, were harvested for depth filtration using a 270-cm2 Clarisolve 20MS-Millistak POD polypropylene and cellulose/DE filter, a X0HC 270-cm2 Millistak POD polypropylene filter, and a 3 × 20 cm2 AcroPak 20 cartridge with a Supor EKV membrane made of hydrophilic polyethersulfone (Pall Corporation) with a pore size of 0.2 µm.
Small-scale depth filtration reveals an upscalable down-scaled model. Large-scale depth filtration can be evaluated reliably through laboratory-scale models based on an ensemble of corresponding small filter systems from the same suppliers. Figure 1 shows a depth filtration using a 270-cm2 Millistak POD filter series to clarify a 5-L cell culture within 3.5 hours, with results normalized to 1 m2 EFA. By achieving the maximum predefined overpressure of 1 bar without filter blocking, the downscale model shows optimal filtration areas for an economical process. Based on these results, we calculate a minimum filter area of 6.2 m2 and 5.1 m2 for 1,000-L cell culture for a Clarisolve 20MS-Millistak POD filter and a X0HC Millistak POD membrane, respectively.
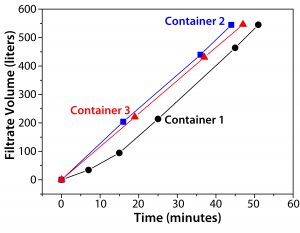
Figure 2: Depth filtration was performed on a MAb-producing recombinant CHO cell (20-µm diameter) suspension containing fed-batch culture supernatant from a 2,000-L Xcellerex XDR-2000 single-use bioreactor (GE Healthcare) with a final cell concentration of 1.38 × 107 mL–1 and 74% viability. Three consecutive depth filtration runs used a 3M Zeta Plus encapsulated three-filter system with a respective 16EZB large filter holder consisting of a 1.6-m2 cellulose/DE filter with a pore size range of 1.5–10.0 µm, a second 1.6-m2 cellulose/DE filter with a pore size range of 0.2–2.0 µm, and a 1.56-m2 hydrophilic PES microfilter with a pore size of 0.2 µm. Clarified culture supernatants were harvested in 1,000-L Mobius single-use process containers made of PureFlex film (Millipore Sigma).
Large-scale depth filtration systems operate with high filtration performance throughout an entire harvesting process. For routinely increasing operational safety, cells are sedimented for 12–22 hours at 10 °C before depth filtration. Figure 2 shows the final three representative processes of a depth filtration for 2,000-L volume from a high–cell-density culture supernatant of a fed-batch cultivation. Every filtration run harvested filtrate of about 500 L of culture supernatant. The graph shows an identical slope for each run at a constant flow rate of 12–13 L/min. For the first filtration (into Filtrate Container 1) the flow rate increased gradually from 5 L/min to 12 L/min within the first 15 minutes of operation. No reduction in performance was observable during the whole clarification process using EFAs of 1.6 m2 and 1.56 m2, with 2,000 L harvested in about three hours.
A Bright Future
A number of single-use technologies for harvest clarification — centrifugation, TFF, and depth filtration — have different advantages and disadvantages (Table 2). We find depth filtration to be highly preferable because of its low investment cost, good scalability, easy handling, and reproducible clarification efficiency. Process improvement is ongoing through optimization of primary recovery without time-consuming sedimentation steps. For example, harvest material may be pretreated with flocculants (such as polydiallyldimethylammoniumchloride) or acidification (11, 12). Meanwhile, suppliers are developing depth filters for special flow-through, further strengthening the value of this single-use harvesting technique.
References
1 Singh V. Disposable Bioreactor for Cell Culture Using Wave Induced Agitation. Cytotechnol. 30, 1999: 149–158.
2 Axelsson H. Cell Separation, Centrifugation. Encyclopaedia of Bioprocess Technology. Flickinger M, Drew S, Eds. Wiley VCH: Weinheim, Germany, 1999: 513–531.
3 Abraham S, et al. Strategies for Improving Mammalian Cell Clarification. Abstr. Pap. Am. Chem. Soc. 225, 2003: BIOT-119.
4 Hanle D. Centrifuges, Animal Cells. Encyclopaedia of Bioprocess Technology. Flickinger M, Drew S, Eds. Wiley VCH: Weinheim, Germany, 1999: 553–559.
5 Kempken R, Preissmann A, Berthold W. Assessment of a Disc Stack Centrifuge for Use in Mammalian Cell Separation. Biotechnol. Bioeng. 46, 1995: 132–138.
6 Schmidt M. Antibody Degradation (Disulfide Reduction) in CHO Production Process. Antibody Development and Production conference. IBC Life Sciences: Westborough, MA, 2009.
7 Liu HF, et al. Recovery and Purification Process Development for Monoclonal Antibody Production. MAbs 2, 2010: 480–499.
8 Fiore J, Olson WP, Holst S. Depth Filtration. Methods of Plasma Protein Fractionation. Curling J, Ed. Academic Press: London, UK; New York, NY; 1980: 239–268.
9 Yigzaw Y, et al. Exploitation of the Adsorptive Properties of Depth Filters for Host Cell Protein Removal During Monoclonal Antibody Purification. Biotechnol. Prog. 22, 2006: 288–296.
10 Shukla A, Gottschalk U. Single-Use Disposable Technologies for Biopharmaceutical Manufacturing. Trends Biotechnol. 31, 2013: 147–154.
11 Brodsky Y, et al. Caprylic Acid Precipitation Method for Impurity Reduction: An Alternative to Conventional Chromatography for Monoclonal Antibody Purification. Biotechnol. Bioeng. 109, 2012: 2589–2598.
12 McNerney T, et al. PDADMAC Flocculation of Chinese Hamster Ovary Cells: Enabling a Centrifuge-Less Harvest Process for Monoclonal Antibodies. MAbs 7, 2015: 413–427.
Stefan R. Schmidt is vice president of process science and production, Stefan Wieschalka is senior scientist in process science, and Roland Wagner is head of production at Rentschler Biotechnologie GmbH, Erwin-Rentschler-Str. 21, 88471 Laupheim, Germany.

