This month, Lester Taylor, director of LC-MS product marketing, and Ning Tang, senior application scientist (both at Agilent Technologies) talk about the advances that have extended adoption of mass spectrometry (MS) in QA/QC laboratories. Ideal technologies for analyzing large proteins and peptides include high-resolution LC-MS time-of-flight (TOF) spectrometry and hybrid LC-MS quadrupole TOF (Q-TOF) methods. The experts also discuss software tools for MS analysis, which facilitate the processing of complex data sets. Traditional biopharmaceutical applications for MS include protein molecular-weight…
Friday, November 1, 2013 Daily Archives
Delivering Affordable Biologics from Gene to Vial
Miriam Monge (from Biopharm Services) revisits her March 2010 article (coauthored with Andrew Sinclair) in an audiocast with managing editor Maribel Rios. She reviewed the industry’s economic challenges at that time, which included costs associated with treatment, increasing market competition, and the rising demand for approved biosimilar products to offset healthcare costs. They wrote, “Many issues the biopharm sector faces appear to be the consequence of a maturing industry with structural impediments to its development brought about by regulation, political…
Monoclonal Antibody Purification with a High Capacity Protein A Resin
“Protein A resins constitute a substantial cost in state-of-the-art mAb purification processes. Factors such as operating cycles, capacity, and mAb titer can have an impact on total costs associated with mAb purification. The purification of a monoclonal antibody from crude feed stock using TOYOPEARL® AF-rProtein A HC-650F, a high capacity protein A resin from Tosoh Bioscience, show that this particular high capacity protein A resin delivers highly pure antibodies at yields approaching 90%.”
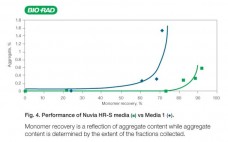
Introducing Nuvia HR-S Chromatography Media
Nuvia HR-S media is a new strong cation exchanger that has been optimized for particle size and chemistry that provides exceptional resolution and high recovery. Nuvia HR-S media demonstrates fast mass transfer kinetics, excellent flow characteristics, and robust chemical stability against common caustic cleaning protocols. Its excellent scalability gives process developers the confidence that results obtained on the bench will be reproducible for large-scale downstream manufacturing. Nuvia HR-S media is the preferred solution for intermediate and final polish applications where…
TOYOPEARL AF-rProtein A HC-650F Host Cell Protein Removal
The following paper compares the host cell protein (HCP) removal capabilities of TOYOPEARL AF-rProtein A HC-650F, TOYOPEARL AF-rProtein A-650F, and another commercially available high capacity protein A resin.
An environmental life cycle assessment comparison of single-use and conventional bioprocessing technology
Single-use technologies offer an attractive option for biopharmaceutical manufacturing, but their environmental impact needs be considered. This paper documents the findings of a Life Cycle Assessment (LCA) study comparing single-use and conventional bioprocessing technology for the production of monoclonal antibodies (MAbs). The study examines the life cycle environmental impacts at 100 L, 500 L, and 2000 L. The results presented focus on the 2000 L production scale. This assessment was reviewed by a third-party review panel. The results demonstrate that…
Biosimilar Products
The Chemistry, Manufacturing, and Controls (CMC) Strategy Forum held on 22 January 2012 in San Francisco, CA, focused on selected scientific and regulatory aspects in the development of biosimilar products. Such products are an increasingly important area of interest for both the biopharmaceutical industry and its regulatory agencies. Biosimilars are highly complex, so scientists have been unable to demonstrate identity to a level typically possible for small molecules. Consequently, specific scientific and regulatory approaches are required to ensure the high…
Robots in the Laboratory
Whether cell-based or molecular biology focused, most assays performed in biopharmaceutical laboratories involve liquid solutions. Increasingly, automated liquid handlers (laboratory robotics) are demonstrating utility in these labs, especially for high-throughput screening and optimization of cell culture media, chromatography conditions, formulations, and so on. Some experts say that screening 100,000 samples/day will soon become routine. But the robots haven’t condemned all manual pipettes to the trash heap — far from it. With multichannel and electronic pipettes improving throughput and reproducibility of…
Process Improvements Increase Production Capacity of a Legacy Product
Implementation of postlicensure process improvements in the biopharmaceutical industry can benefit patients and drug manufacturers alike. Here we demonstrate through a case study how a change to the cell culture medium and process can be taken from proof of concept through scale-up to demonstration of feasibility. We further illustrate the scope and complexity of implementing a change in commercial manufacturing to realize significant benefits such as increased production capacity over an existing legacy process. The Importance of Postapproval Improvements Drug…
Impact of Process Interruption on Virus Retention of Small-Virus Filters
Manufacturers of biopharmaceuticals using mammalian cell culture must have processes in place to minimize the likelihood of virus contamination of their products. Regulatory agencies provide guidelines for testing strategies and best practices to assure raw-material safety and control of the manufacturing process. Safety assurance relies on an interdependent matrix of managed risks, including characterization and control of raw materials, extensive testing of process intermediates, and demonstration of the virus removal capabilities of purification unit operations Figure 1: () A dedicated…