Over the past decade, monoclonal antibodies have become mainstream therapeutics for treating a broad range of conditions from autoimmune disorders to cancer. Part of this evolution is increasing time and cost pressure on biopharmaceutical companies to bring new drugs to market 1, 2. Additionally, companies now routinely engineer and screen molecules for developability and manufacturability during discovery before selecting a final candidate molecule. The biosimilar development paradigm also demands significantly more bioanalytical analysis during initial cell-line and process development. Thus, a significant investment is being made to assure that molecules entering the pipeline are successful. It is therefore imperative to take an integrated approach to cell-line development and early stage screening and process development. This enables companies to make appropriate, timely decisions about pursuing therapeutic candidates for preclinical and clinical manufacturing.
Cook Pharmica LLC and Selexis SA have allied to combine their key strengths for meeting industry needs and to shorten overall timelines. These capabilities include generation of stable and high-performance cell lines at Selexis to rapid bioprocess development, scale-up, cell culture production, downstream processing, and drug-product manufacturing and packaging at Cook Pharmica. Important in helping clients is a seamless project management effort linking all work between contract facilities. The goal is to accelerate delivery of antibody therapeutics to clinics for study and ultimately for sale.
A critical part of biopharmaceutical development is choosing the right cell line with the objective of confirming cell productivities, cell-line stability, and final-product quality. For a proof-of-concept program outlined herein, Cook Pharmica and Selexis were able to successfully develop a productive cell line, design an efficient process, and scale up a commercially available antibody. Selexis generated the high performance SURE CHO-M cell line that was used in Cook’s rapidly scalable development process at its state-of-the-art CGMP biologics manufacturing facility in Bloomington, IN. Current data from the program demonstrate comparability to the originator drug while achieving commercial-ready titers up to 4 g/L.
Cooperative Process Development
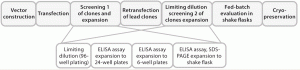
The platform begins with the development of a high-producing CHO cell line expressing the desired target protein 3. We began with a CGMP-compliant host cell line cell line derived from CHO-K1 cells from the American Type Culture Collection (ATCC), adapting it to grow in a chemically defined (CD) culture medium from Irvine Scientific (BalanCD Growth A).
In most cells >80% of genes are switched off or silenced by tightly packed chromatin structures in the
nuclei. So a major challenge of recombinant protein expression is successful integration of recombinant genes into permissive chromatin areas where transcription is not silenced — while also preventing epigenetic gene silencing. We used Selexis Genetic Element (SGE) DNA sequences to maintain transcription at the maximum level. SGE sequences control the dynamic organization of chromatin within mammalian cells to allow for higher and more stable expression of recombinant proteins. SGEs function by insulating nearby genes from the silencing effects of surrounding chromatin, thus increasing expression dependent on copy number and independent of position. The DNA was delivered by electroporation to improve transfection efficiencies.
We screened clones based on their IgG expression titers, then transferred them to shake flasks and further expanded the cultures through a fed-batch process to identify 10 qualified clones. We analyzed their expression constructs at the nucleic-acid level (genetic stability) using quantitative polymerase chain reaction (qPCR), finally selecting a single clone for further process development.
Figure 1 is a schematic of this cell-line development process.
Moving into process development, we performed a media screening study to evaluate a broad set of commercially available cell-culture media with different feed options. We evaluated media types in 14 shake-flask studies with 100 mL working volume for both basal and feed media, total feed amount, feed duration, and temperature shift. Subsequently, we ran two sets of four bioreactor runs at 2-L scale to further evaluate and confirm process parameters such as temperature shift (TS), pH, and glucose levels. Later, we scaled up our 2-L process to demonstrate scalability in several 20-L bioreactor runs with Sartorius-Stedim Biotech BB30 stainless steel bioreactors. The 20-L process compares favorably in metabolic profile with that at 2-L scale, although the titer flattened out at about 2 g/L — whereas in the 2-L process viability declined slower and titer continues to rise beyond 2.5 g/L (
Figure 2).
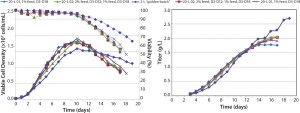
Figure 2: Cell culture process scale-up; (left) viable cell density (VCD) and viability; (right) titer
We developed a standard three-column purification process starting with protein A capture followed by cation exchange in bind–elute mode followed by an anion exchange in flow-through mode. Every step in this downstream process was supported by detailed analysis: e.g., N-glycan analysis to demonstrate comparability and quality and size-exclusion chromatography (SEC) and capillary isoelectric focusing (cIEF) to confirm product quality based on size-variant and charge-variant profiles. After the three column steps, antibody purity was 99.8%, with host-cell proteins (HCPs) at <0.05 ng/mg and an overall yield of 77%.
Because this molecule is a biosimilar, its glycosylation profile is an important critical quality attribute (CQA) under the quality-by-design (QbD) paradigm.
Figure 3 compares N-glycans released from a sample of the innovator product (black) and material from our typical 20-L process. As those results indicate, glycoforms present in the innovator’s molecule are also present in the material produced in this study. In particular, the relative afucosylation contents of both products are very comparable. Afucosylation is an important CQA for antibodies, which can be vulnerable to its binding affinity toward Fcγ receptors on effector cells. That binding leads to clearance of target cells through antibody-dependent, cell-mediated cytotoxicity (ADCC) activity.
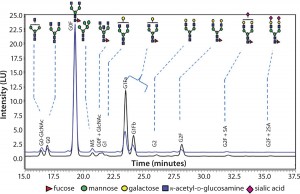
Figure 3: Comparing N-glycans released from innovator product (black) and Cook Pharmica’s 20-L run (blue)
A Successful Partnership
In summary, we demonstrated an integrated cell-line development with process development and scale-up as a successful model for expediting early phase development of antibodies over a nine-month period. This approach did not compromise on the scientifically sound, systematic approach with design-of-experiments (DoE), but rather involved a targeted set of experimental studies to meet the product quality requirements and process scale-up needs.
Together, Selexis SA and Cook Pharmica LLC successfully demonstrated this approach using a commercially available antibody as a model molecule for scale-up from 2-L bench scale through a 20-L pilot-scale process that is scalable to larger GMP operations for clinical production. As our article in review details 3, the resulting data confirm this value proposition to create a reliable process for transfer and scale-up of a high-expressing, stable clonal cell line through a technology platform approach to yield commercial-ready titers with minimal development. Such cooperative alliances will be critical in the future to delivering new treatments for patients as safely and quickly as possible.
Acknowledgments
The authors acknowledge the cell culture, purification, and analytical teams at Cook Pharmica, as well as scientists at Selexis SA, for providing assistance in conducting this study.
Author Details”
Kumar Dhanasekharan, Claudia Berdugo-Davis, Xiaoming Liu, Carl Richey, Zaneer Segu, and Victor Vinci are with Cook Pharmica LLC, 1300 South Patterson Drive, Bloomington, IN, 47402. David Calabrese, Valerie LeFourn, and Pierre-Alain Girod are with Selexis SA, 18 chemin des Aulx, 1228 Plan-les-Ouates, Switzerland.