Clone selection techniques used for development of stable, high-expressing recombinant cell lines suitable for robust fed-batch cell culture processes are critical for biopharmaceutical manufacturing. Basal media screening, feed development and addition strategies, and fed-batch bioreactor performance are all intimately tied to overall performance of the clones during scale-up. Serious issues can arise if a high-quality clone is not established, such as low or unstable protein yield and ineffective use of costly resources.
PRODUCT FOCUS: Recombinant proteins
PROCESS FOCUS: Production
WHO SHOULD READ: Manufacturing, process development, analytical, and product development personnel
KEYWORDS: Cell line development, medium optimization, cell screening
LEVEL: Intermediate
Cell Xpress clone selection technology combines high-speed image scanning and quantitation with high-speed laser manipulation that allows for in situ measurement of recombinant protein secretion of single cells, followed by laser-mediated destruction of the low-producing cells in a population. This involves the LEAP (Laser-Enabled Analysis and Processing) platform from Cyntellect Inc. (www.cyntellect.com) of San Diego, CA (1), and it incorporates an immunofluorescence-based assay for identifying the highest-secreting clones in either transfected or stable pools of cells.
Briefly, cells are seeded into 384-well tissue culture plates in the presence of a matrix designed to capture secreted immunoglobulin G (IgG) molecules. After overnight incubation, captured IgG is detected using a secondary reagent with a conjugated fluorophore, and the viable cells are stained with live-cell tracking dye. Following a short incubation, captured IgG secretions and associated live cells are visualized using the LEAP instrument, and Cell Xpress software is then used to identify and process the highest-producing cells in the population for cloning and expansion.
Following processing, the desired clones are expanded from 384-well plates through 96-, 24-, and 6-well plates for clone characterization and cell banking for future studies. In addition to selection of single-cell clones for expansion, populations that represent a fraction of the total (e.g., the highest 10%, 20%, and so on) can be isolated, making recloning or enrichment of an existing clone a rapid and efficient possibility. Early assessment of existing clones also can be performed to visualize the distribution of single-cell productivity within a given clone.
In this case study, we used the method to isolate several high-secreting clones from a Chinese hamster ovary (CHO) DG44 amplified pool that exhibited considerable clonal secretion variability. Multiple clones were identified as expressing higher levels of recombinant IgG than the initial pool. We then screened each prospective clone in CHO media formulations for growth and productivity characterization in suspension culture. Both clone selection and media formulation screening enhanced productivity, and when used in combination they behaved synergistically, leading to a several-fold improvement in product yield.

WWW.PHOTOS.COM
A clone selection criterion that reduces the number of clones to be evaluated in bioreactor studies is crucial for reducing development costs. In many instances, however, high-quality clones may be terminated prematurely because they do not perform well in the cloning or single-cell expansion media, even though they would perform well once delivered to a more optimal formulation. Such observations support the significance of matching a clone with its correct media formulation early in the selection process to improve the efficiency of process development for biopharmaceutical production.
MethodsThe cell line used in this study was an MTX-amplified CHO DG44 pool producing a human IgG molecule. The cell line was maintained in Medarex’s animal-component–free (ACF) medium formulation supplemented with 4.5-mM L-glutamine and 2-µM methotrexate (MTX). This formulation was the control medium for the study. Growth conditions for spinner flask cultures were a temperature of 36 °C, atmosphere with 7% CO2, and spinner platform speed of 70–80 rpm. Viable cell density was determined by trypan blue exclusion using the Cedex automated cell culture analyzer from Innovatis AG of Bielefeld, Germany (www.innovatis.com). IgG titer was determined on day 14 using a Protein G high-performance liquid chromatography (HPLC) assay.
Clone Selection: Cells were plated in ACF medium at 150–200 cells per well in 384-well C-lect plates from Cyntellect. Following the capture and staining of secreted antibodies, LEAP was used to identify highly secreting cells and eliminate unwanted nonsecreting and poorly secreting cells from each well. Custom software algorithms (2) were then used to automatically locate all viable cells based on green fluorescence, to quantify cell-associated and secreted antibodies based on red fluorescence, and to identify desirable clones based on multiple criteria. Following clone selection, verified clones were scaled up from 384-well cultures to 60-mL volume spinner-flask stock cultures. Those cultures were used for cell bank generation and subsequent studies, as described below.
Medium Screening: We selected four clones for further evaluation in 24 different media from the SAFC Biosciences media library (in comparison with the aforementioned control medium). All were supplemented with 4.5-mM L-glutamine before use. Cells were seeded at 2 × 105 viable cells/mL in 50-mL TPP tubes from Techno Plastics Products AG of Switzerland (www.tpp.ch) with 30 mL of medium and passaged every three to four days. Cultures were maintained at 36 °C, 7% C02, 80% humidity, and 200 rpm. On the third passage, we evaluated the growth kinetics of each cell line. Viable cell densities were determined daily by the Cedex automated cell culture analyzer, and productivity was determined for days seven, 10, and 14 using the Protein G HPLC assay.
Feed Optimization: We evaluated the performance of two clones (B and D) in a small-scale fed-batch process in SAFC Biosciences imMEDIAte Advantage 65658 and 65955 media and compared it with that in the control medium. All media were supplemented with 4.5-mM L-glutamine. Stock cultures were maintained in 125-mL Erlenmeyer shaker flasks from Corning (www.corning.com) by seeding at 2 × 105 viable cells/mL in 30-mL working volumes. The cells were expanded into 1-L shaker flasks in 400-mL working volumes. At mid-stage logarithmic growth (on day 3), 30 mL of pooled culture was divided into 50-mL TPP tubes to initiate the small-scale fed-batch study.
For that study, cultures were maintained at 36 °C, 7% C02, 80% humidity, and at 120 rpm for Erlenmeyer shaker cultures and 200 rpm for TPP cultures. All were fed glucose and L-glutamine to maintain levels of 4–5 g/L and 5–6 mM, respectively. We tested five additional feeds individually and compared them with cultures fed only on glucose and glutamine. One of the five test feeds was added to the cultures on days 5 and 7. Cultures were monitored daily for viable cell counts and viability using the ViaCell assay on a Guava PCA-96 from Guava Technologies of Hayward
, CA (www.guavatechnologies.com). We measured IgG on days 10 and 14 using a Protein G HPLC assay.
IgG Partial Purification: IgG was concentrated from spent cell culture media with a 30-kD Amicon Ultra-15 centrifugal filter unit from Millipore (www.millipore.com) using a swing-bucket rotor at 4,300 rpm for 50 minutes. After concentration, the IgG was purified with Protein A resin from GE Healthcare (www.gehealthcare.com) in a 96-well filter plate from Innovative Microplate (www.innovativemicroplate.com).
Before purification, we used 200 µL of absolute ethanol to wet the filters and removed the excess by centrifugation at 1,600 rpm for three minutes. Each filter-plate well received 100 µL of Protein A slurry by pipette, and storage buffer was removed by centrifugation. Protein A was washed twice by adding 200 µL of wash buffer (20-mM citrate, 150-mM NaCl, pH 7.0 ± 0.2), shaking for a minute, and removing the buffer by centrifugation. We then added 300 µL of the concentrated spent cell culture media to each well containing Protein A resin and shook the filter plate for 10 minutes. Plates were centrifuged to remove media and then washed twice as described. Then we added 200 µL of elution buffer (25-mM citrate, pH 3.0 ± 0.2) and shook the filter plate for another minute. After centrifugation, eluent containing the IgG was collected from the filter plates. IgG concentration was measured by A280 spectrophotometry on a NanoDrop ND-1000 (www.nanodrop.com), and samples were diluted to a standard concentration of 1 mg/mL in elution buffer.
Cation-Exchange (CEX) HPLC: We fractioned 25 µg of partially purified IgG based on charge heterogeneity using a 4.6-mm × 250-mm ProPac WCX-10 weak cation-exchange column from Dionex (www.dionex.com) connected to an Alliance HPLC with PDA detection from Waters (www.waters.com). Mobile phase A consisted of 10-mM sodium phosphate (pH 7.5 ± 0.2), and mobile phase B consisted of 10-mM sodium phosphate and 100-mM sodium chloride (pH 7.5 ± 0.2). Separation and detection were performed at 25 °C and 220 nm using an initial gradient of 95%→60% for mobile phase A followed by a wash step of 100% mobile phase B.
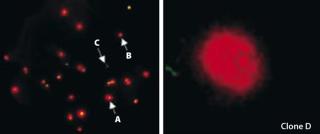
Results and DiscussionCloning was performed in 384-well C-lect plates from Cyntellect using the Cell Xpress assay and software from SAFC Biosciences. Figure 1 (left panel) shows a Cell Xpress image of the initial DG44 pool. From this initial population, we identified and successfully grew out eight clones from two separate 384-well plates. These clones were expanded to spinner-flask cultures (60-mL volume), and frozen banks were generated 10–12 weeks after cloning.
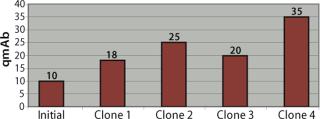
Of the eight clones generated, five exhibited increased specific productivity (qP) when compared with the initial cell line, whereas the remaining three maintained the same titer and qP as the initial line. Initial qP was 10–12 p/c/d (in batch spinner cultures). Among the five clones that showed improvement, we observed 15–40 p/c/d under identical growth conditions (Figure 2).
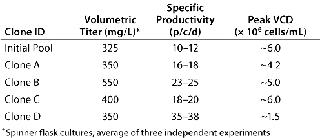
Volumetric titer of the initial line was 300–350 mg/L (in batch spinner cultures), and among the eight clones generated four showed an equal or greater volumetric productivity when grown under identical conditions (Table 1). But clone D, which had the highest qP, grew to a relatively low peak viable-cell density, suggesting a potential for increasing volumetric titer by improving growth rate (Table 1). Interestingly, that clone expanded slowly following Cell Xpress selection, but it was maintained because of its superiority in visualized single-cell secretion (Figure 1, right panel). Overall, we observed a 20–60% increase in volumetric titer when comparing these clones with the initial pool.
Table 1: Summary of growth and productivity for selected clones
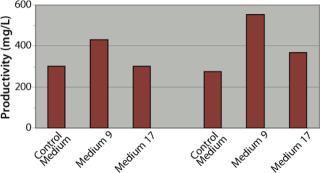
Medium Screening: Based on the reduced peak cell densities observed for the clone with the highest qP in our growth curve assays, we performed a media screen on the four clones to optimize cell growth and increase volumetric titers. We passaged the clones twice in 24 different formulations from the SAFC Biosciences CHO media library before performing growth-curve experiments in TPP tubes. Two media (#9 and #17) consistently demonstrated superior performance, so they were selected for further studies using clones B and D (Figure 3).
In those studies, clones B and D yielded similar productivity levels of 275–300 mg/L when cultured in the control medium using a batch process. Clone B exhibited a 44% increase in batch productivity when cultured in Medium 9 to 432 mg/L b
ut no increase when using Medium 17. Clone D yielded much greater increases in productivity than clone B when cultured in either medium: specifically, a 100% increase in Medium 9 (552 mg/L) and a 34% increase in Medium 17 (368 mg/L). Overall, the highest batch productivity titer achieved was that of clone D cultured in Medium 9, with its titer of 552 mg/L. These data highlight the significance and benefit of pairing specific media with specific clones, even if the clones are derived from the same parental clone or pool.
Fed-Batch Development: In the experiment illustrated by Figures 4 and 5, clones B and D were tested in a small-scale fed-batch process, comparing their productivity in the control medium, Medium 9, and Medium 17. We tested five feeds in combination with glucose and glutamine feeds. Feeds 1 and 2 were complete feeds based on media composition; that is, they contained multiple nutrient groups of amino acids, vitamins, trace elements, growth factors, and lipids. Feeds 3, 4, and 5 were component feeds containing only one or two component groups within each.
By feeding only glucose and glutamine, clone B achieved significant improvements in productivity of 83%, 23%, and 52% when cultured in control medium, Medium 9, and Medium 17, respectively, over nonfed cultures. Overall titers achieved in these three media when fed with glucose and glutamine were 530–550 mg/L and were not significantly different by comparison, although Medium 9 clearly outperformed both the control medium and Medium 17 under identical batch culture conditions.
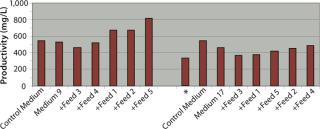
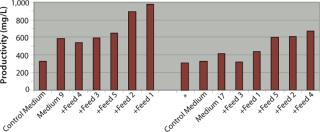
Our performance ranking for the five feeds was very different when comparing Medium 9 and Medium 17, and also when comparing clones B and D. When clone B was cultured in Medium 9 using glucose and glutamine feeds in combination with Feeds 1, 2, or 5, titers increased by 24%, 25%, and 34% respectively over fed-batch cultures in control medium or Medium 9 alone (batch). Both complete feeds (Feed 1 and Feed 2) yielded significant improvements in productivity titers, and only one of the component feeds (Feed 5) yielded any significant improvement, although it was the greatest improvement. The highest titer achieved using clone B was 817 mg/L, which was achieved by using Medium 9 in combination with Feed 5. For clone B in Medium 17, none of the five feeds tested in combination with glucose and glutamine yielded improvements in titers over the control fed batch.
Productivity titers of clone D were less affected than those for clone B by feeding only glucose and glutamine, with increases in titers of only 19%, 6%, and 13% in control medium, Medium 9, and Medium 17, respectively. However, by contrast with the feed performance for clone B, we identified multiple feeds that enhanced productivity over the control fed batch for clone D cultured in both Medium 9 and Medium 17. In Medium 9, the best-performing feeds were the complete feeds (Feed 1 and Feed 2), yielding productivity titers of 979 mg/L and 889 mg/L respectively — increases of 200% and 172% over the control medium fed-batch titer of 326 mg/L. In Medium 17, the best-performing feeds were Feed 2 (a complete feed), Feed 4 and Feed 5 (both component feeds), reaching titers of 609 mg/L, 670 mg/L, and 599 mg/L, respectively — increases of 87%, 105%, and 84% over control titers.
So Clones B and D achieved very different productivity titers in small-scale fed-batch processes depending on the selection of base medium and feed used (Table 2). The best titers achieved by both clones were not equal, with clone D achieving a titer of 979 mg/L and clone B achieving a 17% lower titer of 817 mg/L under the best combination of medium and feed. Although the best medium tested for each clone was Medium 9, the best feeds were different, with the best for clone B being Feed 5 and that for Clone D being Feed 1.
Table 2: Summary of fed-batch results
Cation-Exchange HPLC of IgG Produced in Different Media Formulations: A major concern for the biopharmaceutical industry is maintaining the product quality parameters of recombinant proteins such as monoclonal antibodies when process changes occur during product development. Changes in the biochemical profile of a protein product could affect its therapeutic or pharmacokinetic properties and require repeating preclinical or clinical studies. Because charge heterogeneity is commonly observed with recombinant IgGs, we used cation-exchange (CEX) HPLC to evaluate changes in product quality that might result from changing media formulations. Three distinct resulting chromatographic patterns were observed when clone D was produced in the 24 different formulations during media screening studies. Figure 6 shows representative chromatograms of those different profiles.
Charge heterogeneity can result from multiple causes such as deamidation, oxidation, isomerization, amino acid changes, sialic acid addition to glycoforms, N-terminal pyroglutamic acid, or C-terminal lysine clipping. We did not elucidate the cause of the heterogeneity represented by the various peaks in these chromatograms. However, these results clearly
demonstrate differences in the relative quantity of peaks and the complexity of the chromatographic profiles for IgGs produced in different media. This illustrates the need for careful monitoring of product quality during process development.
Cell Xpress technology greatly facilitates clonal cell line generation. Compared with traditional methods such as limiting dilution, single-cell clones derived in this way exhibit superior or comparable frequencies of high productivity and can be developed in a shorter period of time. The technology also provides a powerful approach to analyzing population dynamics of cell lines producing therapeutic proteins by examining r-IgG secretion at the single-cell level.
Of keen interest was our ability to identify very slow growing but high-producing clones and screening the media conditions that would improve their growth rates to develop an acceptable production cell line. Using traditional cell line generation approaches, clones such as these would not meet criteria regarding generation time necessary for expansion beyond the 96-well stage. Because early visualization identifies these clones as high producers, the potential for growth rate improvement through medium optimization can be considered during early expansion and clone characterization.
Our results demonstrate that clones can yield significantly different productivity titers under the same nutrient conditions and that the same feed can perform very differently depending on the selection of base medium used for culture. Furthermore, it is clear that feed performance can also depend on the specific clone used in a process, with different clones demonstrating different feed preferences based on performance ranking and overall titers. The best recombinant protein titers will be realized by combining clone selection with process optimization, including clone-specific media and feed selection.