Recombinant therapeutic protein production using cell culture systems is a US$70 billion market. Most biotherapeutic proteins, including monoclonal antibodies (MAbs), are produced in Chinese hamster ovary (CHO) cells, which can generate the posttranslational modifications required for full biological function. Single-cell cloning is an important step in generating homogenous recombinant protein-producing mammalian cell lines. Recent advances in media development technologies have enabled limiting dilution cloning (LDC) and protein production in a serum-free environment to meet regulatory requirements.
LDC allows homogeneity of a selected cell line. It involves diluting a pool of transfected cells down to a single cell per culture well and then expanding that single cell to a larger cell population called a clonal cell population or cell line. Success of this process requires a medium containing correctly balanced nutrients that promote cell growth at extremely low cell densities. To meet stringent regulatory requirements, most current cell culture production processes are performed using well-defined serum and protein-free media (1), whereas development of cell lines is still performed with serum-containing medium. That requires adaptation of cells to serum-free media after clonal selection.
PRODUCT FOCUS: PROTEINS, ANTIBODIES
PROCESS FOCUS: UPSTREAMPROCESSING
WHO SHOULD READ: PROCESSDEVELOPMENT AND FORMULATION
KEYWORDS: CHO CELLS, CLONING, GROWTH MEDIA, CLONING MEDIUM, TRANSFECTION, IGG
LEVEL: INTERMEDIATE
Puck et al. first reported clonal growth of mammalian cells at high plating efficiencies in large volumes of cell culture media (2), and other investigators have confirmed their results (3). Clonal growth required media containing whole serum, dialyzed serum, purified serum components, or undefined dialyzed components from the serum (4,5). Ham reported the first successful experiments of clonal growth with a completely synthetic medium, F-12 (3). However, that medium failed to perform reproducibly at low cell densities. MCDB 301 was subsequently developed specifically for clonal growth of CHO cells, and investigations have shown that supplementation of MCDB 301 with insulin or methylcellulose is required for Chinese hamster lung cell growth in low densities (6). Several companies have developed chemically defined and animal-origin-free (AOF) media formulations for CHO cell growth (7) and bioprocessing applications (8). Here, we compare the performance of various chemically defined (CD) serum-free media in LDC assay, clone expansion, and other parts of the bioprocessing work f low.
Materials and Methods
Materials: We obtained all cell lines, cell culture media, supplements, serum, and transfection kits from Life Technologies Corporation, unless otherwise indicated. We purchased EX-CELL cloning medium and 96-well plates from Sigma and Fisher Scientific, respectively.
Cell Culture: Stock cultures of test cells lines — parental CHO-S, recombinant CHO DG44-erythropoietin (EPO) clone, and CHO-S or CHO DG44 pools transfected with an IgG or EPO expression plasmid — were maintained in suspension culture in CD CHO, CD OptiCHO, or CD FortiCHO growth media. We maintained cells requiring selection agents posttransfection for at least three passages without selection before cloning. We used basal medium Eagle (BME) with Earle’s salts supplemented with 10% fetal bovine serum (FBS) as a positive control for comparing performance of AOF cloning medium. We supplemented all cell growth media with 8 mM L-glutamine and grew cells in an incubator set at 37 °C, 85% humidity, and 8% CO2.
Preparation of Conditioned Medium: We seeded cells at 3×105 viable cells/mL in a sufficient culture volume of different growth media supplemented with 8 mM L-glutamine to generate enough conditioned medium needed for cloning experiments. Cells were grown for four or five days and were centrifuged at 800g for five minutes. We collected supernatants (conditioned medium), sterilized them using 0.22-µm membrane filters (SteriFlip, Millipore), and used them in cloning assays. Aliquots were frozen at –80 °C until needed.
Transfection: Parental CHO-S cells (1×107 cells/mL) were transfected with 50 µg of IgG expression plasmid using FreeStyle MAX CHO expression system (Invitrogen) following manufacturer’s recommendations. We generated stable pools using simultaneous selection with puromycin and methotrexate (MTX) at a 500-nM concentration for five weeks. We selected three pools resistant to MTX and used them for LDC assay using 14 96-well plates for each pool.
Cloning Protocol: We grew cells in respective growth media for at least three passages in a three- to four-day subculture schedule before performing a cloning assay. We determined viable-cell densities using trypan blue exclusion and a Vi-Cell (Beckman Coulter) analyzer. Cells were serially diluted to 1,000 cells/mL in growth media.
Using a previously established cloning assay (9), we placed 38.4 mL of cloning medium in a 50-mL conical tube and prewarmed it for one hour in an incubator set at 37 °C, 85% humidity, and 5% CO2 with the cap loosened. We added glutamine to the prewarmed media to 6-mM final concentration and mixed gently. We added 200 µL of serially diluted cell stock (1,000 cells/mL) to this medium to reach a final dilution of 5 cells/mL in a volume of 40 mL. We mixed the contents gently by inverting tubes five to six times and dispensed 200 µL of this media in each well of three 96-well plates.
The plates were then incubated for 14 days at 37 °C, 85% humidity, and 5% CO2. We observed those plates on day 14 using a microscope and counted the wells with positive cell growth to calculate cloning efficiencies. We adjusted the initial volume (38.4 mL) of cloning medium accordingly when we added hypoxanthine and thymidine (HT) supplement (1:100 v/v) or conditioned medium (10% v/v) during cloning. Growth media, conditioned media, and cloning media were matched in each cloning experiment. Each media combination assay was performed in triplicate. We used a serum free EX-CELL cloning medium (Sigma) supplemented with 4 mM L-glutamine and BME supplemented with 10% FBS and 6 mM glutamine for comparing cloning efficiencies.
Clonal Expansion and Productivity Assay: To demonstrate that the CHO-S clones obtained from either AOF cloning medium or CD FortiCHO are expandable and that our cloning process using those media is capable of comparable cell growth and productivity, we selected more than 100 recombinant CHO-S clones producing IgG from each media and expanded in CD FortiCHO supplemented with 8 mM L-glutamine. We performed primary screening starting with 96-well plates followed by 24-well, six-well plates, and bioreactor tubes or shake flasks. We carried out secondary screening in bioreactor tubes or six-well plates with 3×105 cells/
mL seeding density.
We measured IgG expression on the fifth day after seeding. We ranked all clones on the basis of IgG production and selected the 28 top-producing clones for tertiary screening. We grew cells in CD FortiCHO medium containing 8 mM L-glutamine and Anti-Clumping Agent (Gibco) (1:1000 v/v) using simple fed-batch culture. We added 4 g/L glucose to those cultures on days three and five so that glucose did not become rate limiting during growth. A paired two-sample student t-test was used to determine the statistical significance (p ≤ 0.05) of our data.
Results and Discussion
Our fundamental finding is that successful generation of clones derived from a single cell depends on cell type, medium used, presence or absence of additional supplements, and the recombinant protein expressed. Comprehensive medium screening and cloning assay protocol optimization in this study resulted in serum-free and CD-media options that are suitable for supporting single-cell cloning by limiting dilution.
To assess the suitability of a lean, AOF cloning medium and three CD growth media formulations for single-cell cloning, we used a CHO-S parental cell line, a recombinant CHO DG44 cell line expressing EPO, and CHO-S or CHO DG44 cell pools transfected with either IgG or EPO expression plasmids. We used EX-CELL cloning medium supplemented with 4 mM L-glutamine and BME supplemented with 10% FBS and 6 mM glutamine for comparing cloning efficiencies among those media formulations.
Cloning efficiency of lean AOF cloning medium differs between CHO-S and CHO DG44 cell lines. Traditionally, development of recombinant protein-producing cells was performed using serum-containing medium. Cells had to subsequently be adapted to serum-free media following clonal selection. That process is risky because the selected clones may lose productivity or protein quality during adaptation. It also significantly lengthens the time needed for cell-line development. Cell culture production methods that can be performed in fully chemically defined, protein-free media will meet strict regulatory guidelines and will mitigate the risks mentioned above.
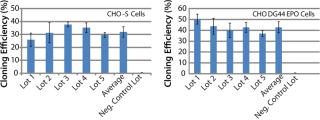
To address those issues, we developed an AOF cloning medium and tested its performance in LDC assays. As a first attempt, CHO-S parental cells and recombinant CHO DG44-EPO clones were used in the assay to control for variation (Figure 1). Results from those assays revealed consistent performance for different lots of media (p > 0.05). The observed minor differences are because different stock of cells were grown at different times. Recombinant CHO DG44-EPO cells consistently showed 10% higher cloning efficiencies compared with CHO-S parental cells under identical conditions. Results also showed that the performance of AOF cloning medium was equal to that of EX-CELL cloning medium and 70% of the cloning efficiency of BME supplemented with 10% FBS (data not shown). Having established the performance of AOF cloning medium using parental CHO-S or CHO DG44 EPO clone, we carried out additional experiments using CHO-S or CHO DG44 cell pools transfected with either IgG or EPO expression constructs (Figure 2). CHO-S parental cells or recombinant CHO-S-IgG pools showed high cloning efficiencies using AOF cloning medium.
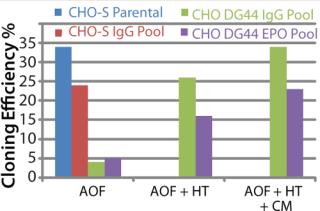
By contrast, CHO DG44-IgG or EPO transfected pools showed poor cloning efficiency in the absence of supplement addition. We added either HT supplement alone or HT supplement combined with conditioned medium (generated from CHO DG44 cells transfected with empty pOptiVEC plasmid vector) during cloning to examine whether cloning efficiencies improved in recombinant CHO DG44 pools. Addition of HT supplement produced five- and threefold improvement, and addition of both HT and conditioned medium gave seven- and fourfold increase in cloning efficiencies in CHO DG44 cells transfected with IgG and EPO, respectively. Removal of selection pressure several passages before cloning further improved cloning efficiencies in CHO DG44 pools (data not shown).
CD growth media do not require supplements for CHO-S cells or CHO-S transfected cell pools. The lean AOF cloning medium did not perform well with CHO DG44 pools without supplements. We evaluated three chemically defined CHO growth media to examine whether they can be used for LDC assay using parental CHO-S cells, a recombinant CHO DG44-EPO clone, and CHO DG44 cell pools transfected with either EPO or IgG expression constructs.
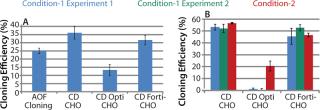
Experiments using CHO-S cells and pools with different growth media showed 44% and 26% higher cloning efficiencies with CD CHO and CD FortiCHO, respectively. CD OptiCHO showed 50% lower efficiency compared with AOF cloning medium (Figure 3A).
Experiments conducted with CHO DG44-EPO cells showed similar performance among AOF cloning medium, CD CHO, and CD FortiCHO media, both when the latter two were used as cloning media and as growth media. However, CD OptiCHO failed when used as a cloning medium (Figure 3B) but showed significant improvement (p = 0.012) when AOF cloning medium was used for LDC after cells were grown and diluted in CD OptiCHO.
We repeated experiments using recombinant CHO-S-IgG pools, and results revealed high cloning efficiencies when we used CD growth media as growth and dilution media or cloning media (Figure 4LEFT). Addition of HT supplement and conditioned medium did not improve cloning efficiency of CD media when used for cloning (data not shown). It can be concluded from these three experiments that either AOF cloning medium, CD CHO, or CD FortiCHO media can be used for successful LDC assays when CHO-S cells, recombinant CHO-S cell pools, or recombinant CHO DG44 clones are used for cloning. However, CD OptiCHO growth medium did not perform well under similar conditions. CHO-S cells, recombinant CHO-S cell pools, or recombinant CHO DG44 cl
ones required no supplementation with HT or conditioned medium.
CHO DG44 cells transfected with IgG or EPO expression plasmids show different profiles. Our studies carried out with recombinant CHO DG44 cell pools expressing either IgG or EPO showed different cloning profiles. When CD growth media or AOF cloning medium were used in LDC assays, cloning efficiency was very low. In CHO DG44-EPO pools, addition of HT supplement and conditioned medium to CD FortiCHO medium and to AOF cloning medium resulted in a two- to threefold increase in cloning efficiencies (Figure 4MIDDLE). We observed moderate improvements with CD CHO and CD OptiCHO media. Similar experiments conducted with recombinant CHO DG44-IgG pools showed that addition of HT supplement and conditioned medium to all CD-growth media or to AOF cloning medium improved cloning efficiencies (Figure 4RIGHT).
Studies conducted to evaluate performance of various CD serum-free media in LDC using different cells showed cell-line–specific results. Performance of media were also sensitive to the protein expressed in the host cell line. Observed variations among different CHO cell populations can be attributed to several factors, including integration site of the recombinant DNA, chromosomal changes induced by foreign DNA integration, and other transcriptional regulatory elements. Primary diploid Chinese hamster (Cricetulus griseus) cells contain 22 chromosomes (10), and parental CHO-K1 cells have 21 chromosomes and nine Z-group chromosomes that are structurally different from normal hamster cells (11).
CHO DG44 strain was generated from CHO cell lines using chemical or radiation-mediated mutagenesis. Both alleles of dihydrofolate reductase (dhfr) on chromosome 2 are deleted, and these cells contain only 20 chromosomes (12). Derouazi et al. investigated the stability and cytogenetic characterization of CHO cells established by different transfection methods and showed single integration site — regardless of the gene delivery method or number of copies integrated (13). It was also observed that the integration site is not specific to a single chromosome. Integration of foreign DNA induced rearrangements on the same chromosome where it is integrated or on different chromosome in 50% of cell lines established.
Results of this work demonstrate that different cell lines and transfected pools require different nutrient composition and/or concentrations to survive and grow at extremely low cell density. The observed specificity may be due to genetic alterations frequently associated with the establishment of recombinant CHO cell lines.
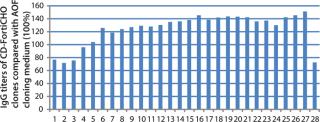
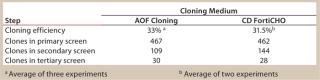
CD FortiCHO medium can be used for entire bioprocess workflow. We conducted additional experiments to confirm that the single-cell clones generated using AOF cloning medium or CD FortiCHO growth medium indeed produced the protein of interest. We isolated and screened ∼460 clones from CHO-S cells transfected with an IgG expression vector. Finally, we selected 30 clones and compared IgG expression levels after tertiary screening in shake flask cultures. Results showed that clones generated from both AOF cloning medium and CD FortiCHO produced similar IgG expression levels. (Figure 5, Table 1). Both AOF cloning medium and CD FortiCHO growth medium support cloning of CHO-S pools. Nonetheless, using CD FortiCHO growth medium is advantageous because the same medium can be used during the entire cell engineering workflow (growing parental cells, transfection, selection, single-cell cloning) and scale-up. This not only saves time, but it also eliminates risks associated with adaptation, including selection against desired traits.
Table 1: Comparison of AOF and CD FortiCHO cloning media (see
Figure 5)
Author Details
Prasad Dhulipala, PhD, is a staff scientist of bioproduction research and development at Life Technologies Corporation, 3175 Staley Road, Grand Island, NY 14072; 1-716-774-3032; fax 1-716-774-6996; prasad.dhulipala@lifetech.com. Margaret Liu, Shannon Beatty, and Michelle Sabourin are staff scientists , Graziella Piras and Shawn Barrett are senior managers, Richard Hassett is project manager, and Stephen Gorfien is senior director, all at Life Technologies.
REFERENCES
ree Single-Cell Cloning Medium for Chinese Hamster Ovary Cell LinesCell Technology for Cell Products, Springer, New York:595-597.