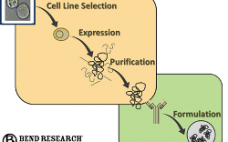
In the spirit of implementing PAT (Process Analytical Technology), biopharmaceutical companies are striving to gain a more fundamental understanding of what is happening to the cells within their bioreactors. Implementation of online tools like Raman and Dielectric Spectroscopy are helping to provide insight, yet there is still a gap integrating this data with off-line measurements such as cell density, viability, metabolite levels and titer. Bend Research, in collaboration with major biopharmaceutical companies, is working to advance MAST, a modular, automated sampling platform that can provide samples directly from bioreactors to analytical devices, while maintaining process sterility. In this webcast, Clint Pepper, Ph.D. and Lisa Graham Ph.D., P.E. of Bend Research focus on this overall cell-level “observability” concept and illustrate how the MAST platform is incorporated.
Manufacturing
Bioreactor Monitoring – Best Practices for Cell Counting, Sizing, and Viability
Manufacturers utilizing bioprocesses for production need accurate and reliable tools for monitoring bioreactors. The key parameter that many bioprocess engineers rely on is viable cell density. This is a compound parameter arising from knowledge of the cellular concentration and the total cellular viability. Viable cell density is the mathematical product of these two primary pieces of information. In order to minimize variability, the detection technique of each of the primary measurements (concentration and viability) must be understood separately.
In this educational webcast, Matthew N Rhyner, PhD., Global Analytical Marketing Manager at Beckman Coulter Inc, discusses two of the most powerful techniques for assessing concentration and viability: the Coulter Principle and flow-based imaging. Join Dr. Rhyner as he compares the strengths and weaknesses of these techniques and discusses competing technologies. The webcast concludes with some recommendations for best practices in monitoring cell count, size, and viability.
Standards for Ancillary Materials Used in Cell- and Tissue-Based Therapies
Cell- and tissue-based therapies are being used increasingly to treat many diseases for which currently no other adequate treatment options are available. These products contain human or animal cells that can replace, regenerate, or augment a recipient’s diseased, dysfunctional, or injured cells, tissues, or organs. Cells or tissues might be unmanipulated, or their biological characteristics can be altered ex vivo before administration of the final product to patients. Examples of cell therapies range from traditional blood transfusions to recent approaches…
Protein A
The number of blockbuster monoclonal antibody (MAb) drugs continues to grow. In 2008, MAbs generated revenues in excess of US$15 billion (1), making them the highest-earning category of all biotherapeutics. The world MAb market will reach $62.3 billion in 2015, with next-generation therapeutic antibody revenues reaching $2.3 billion in 2015 according to Visiongain reports published in September and November 2011 (2, 3). Biosimilar antibodies will also begin to enter established markets as regulatory authorities clear approval pathways for them. Most…
Upstream Chemistry Analysis in Cell-Based Process Development
Cell line selection is important to any pharmaceutical company’s development pathway for biological compounds (1). In cell-line selection laboratories, many different, slightly variable cell lines are tested in parallel for desired characteristics. Candidate cell lines are chosen for further development on the basis of their performance in basic tests of critical quality attributes (CQAs). Historically, such cell lines were selected in large-volume containers because it was necessary to have sufficient volume in culture to allow repeated sampling without damaging the…
Automation of Microbioreactors
Current methodologies in genetics and microbiology enable researchers to influence metabolic pathways of microbial cells in many directions. Beside the academic interest in investigating fundamental functions in metabolic pathways, commercial production of valuable compounds by microbial hosts is state of the art. For example, such products include enzymes (lipases, proteases, phytases), therapeutic agents (insulin, antibodies), bulk chemicals (lysine, glutamate, citric acid), or the microbial cells themselves (used in brewing or milk processing), with therapeutic agents probably the fastest growing market.…
Moving Forward with a Gene Therapy for Damaged Hearts
A cocktail of three specific genes can reprogram cells in the scars caused by heart attacks into functioning muscle cells. Adding a gene that stimulates the growth of blood vessels enhances that effect, say researchers from Weill Cornell Medical College, Baylor College of Medicine, and Stony Brook University Medical Center in a report that appears online in the Journal of the American Heart Association (1). “The idea of reprogramming scar tissue in the heart into functioning heart muscle was exciting,”…
How to Achieve Greater Efficiency in Biopharmaceutical Process Development
Read about GE Healthcare Life Sciences’ support from efficient process development to production through products, consulting services as well as specific, individual collaborations. “Our aim is to help bioprocessing teams to map the optimal plan to transform an idea into results, with greater flexibility and confidence, reducing time and costs along the way. Our global team of bioprocessing experts will support you in the optimization and troubleshooting of existing unit operations or in the design of efficient and cost-effective processes…
Profitability in the Biosimilars Market
The biosimilars space offers significant commercial opportunity. About US$60 billion of branded biologic sales will lose patent protection over the next few years, including some of the largest-selling monoclonal antibodies (MAbs). Companies are jostling among themselves, each seeking the best position to exploit that opportunity. Regulators are creating and refining the necessary pathways to success, alliances are being forged, and companies are being acquired. Despite the significant opportunity for biosimilar MAbs, significant risks remain. Perhaps the most significant of those…
An Analysis of the US Biosimilars Development Pipeline and Likely Market Evolution
No consensus concerning biosimilar-related terminology and definitions has yet been achieved (1,2,3). Biosimilars may be defined as biopharmaceuticals slated for generic-drug–like, abbreviated, comparisons-based approvals through a formal biosimilar approval pathway in the United States, European Union, and/or other highly regulated and developed countries based on a demonstration of substantial (bio)similarity to a reference product. As required in the United States, biosimilar active agents (those involving recombinant proteins) must be identical in primary sequence with their reference products. Analytical and comparative…