The field of gene therapy is in great need for well characterized AAV reference materials, according to viral vector delivery services firm Vigene Biosciences.
Recombinant adeno-associated virus (rAAV) is a widely used gene delivery tool for research and clinical applications in the gene therapy field. To compare the pharmacokinetics and efficacy of rAAV, well characterized AAV reference materials are needed for assays and in-house reference materials calibration. AAV reference materials are also essential for AAV in process development and AAV analytical methods development.
Moreover, per FDA’s gene therapy CMC guidance document released January 2020, commercially sourced, well-characterized AAV reference materials are listed as reference materials utilized in the development of a gene therapy.
What critical quality attributes are needed for AAV reference materials?
- Viral vector genome copies
- Infectious titer
- Full/empty ratio
- Viral particle titer for empty capsids
qPCR-based vector genome titration to measure viral genome copies is utilized to calculate AAV viral vector doses for gene therapies. Reference materials (full capsids) with accurate vector genome concentration can be used in qPCR-based vector genome titrations. Since AAV is known to produce empty vial particles without payload, the full-to-empty ratio of standard materials is also very critical in characterizing rAAV productions. High quality and high purity empty capsid reference materials can be used as reference materials in assays, such as HPLC, ELISA, etc.
Commercially available AAV reference materials
Vigene Biosciences, a Charles River Company, is a leader in viral vector manufacturing and analytic assays. Vigene provides extensively analyzed AAV reference materials with viral particle concentration and full/empty ratio. Six AAV serotypes of reference materials are offered, AAV1, AAV2, AAV5, AAV6, AAV8 and AAV9, full capsids and empty capsids offered for each serotype. We also offer custom production AAV reference materials for large scale applications or a different serotype, using the same production process and release assays.
As current inventory for AAV2 and AAV8 is limited in the industry, rely on Vigene as a trusted source for AAV reference materials.
Explore fully-characterized AAV reference materials
AAV reference materials are produced in HEK293T cells using transient transfection method, purified using iodixanol gradient ultracentrifugation, followed with chromatography purification. AAV viral particles are extensively analyzed with both molecular-based assays and transmission electron microscopy (TEM) for full viral vector characterizations, including full-to-empty ratio. A few thousands of vials per lot are available for each serotype of AAV reference materials.
The AAV standard materials are characterized for purity, viral genome copy/viral particles titer, as well as full/empty ratio. The viral particles are also tested for safety, including endotoxin, bioburden, mycoplasma testing. Our AAV reference materials are quantified based on the reference standard material from ATCC.
TEM Analysis of AAV Reference Materials (Full Capsids)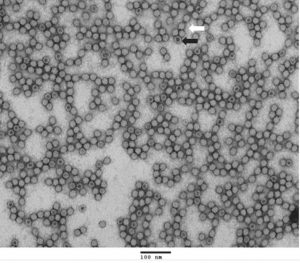
TEM (transmission electron microscope) image of purified AAV9 reference materials (full capsids). Full capsids appear as white spheres (white arrow) while empty capsids appear as spheres with black dots in the center (black arrow).
To learn more about Vigene’s AAV reference materials, visit VigeneBio.com.
