 This webcast features: Mark Fitchmun, President and CEO, Somatek, Inc.
This webcast features: Mark Fitchmun, President and CEO, Somatek, Inc.
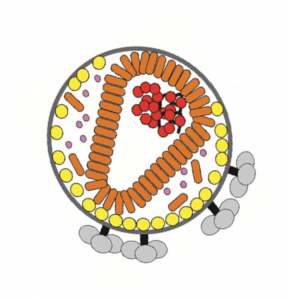
Purification of enveloped viruses and virus-like particles presents several challenges due to their large size and complexity. In this webinar, a case study will be presented and will detail the importance of resin screening, endonuclease treatment, process development, and the scale-up purification of a retrovirus-like particle intended for use in human subjects. The resulting current good manufacturing practice (CGMP) compatible process required approximately four hours to purify 240 L of nuclease-treated bioreactor harvest and resulted in a 99% reduction in process volume and up to 65% recovery of virus particles with a final purity consistent with requirements for clinical trials. This process using Nuvia Q anion exchange chromatography resin (Bio-Rad Laboratories) is readily scalable, fast, low cost, simple, and CGMP compatible and can be used for the capture step in clinical-grade manufacture of retrovirus vectors.
Webcast Overview
- Introducing retrovirus vectors and associated analytical methods
- Discussing scale-down modeling
- Establishing chromatographic parameters
- Examining process scale-up execution and evaluation
This presentation is sponsored by Bio-Rad.
Just fill out the form below to watch the recorded webcast.
