As organizations begin their annual budgeting meetings, the question will inevitably arise: How can we reduce costs and still retain or improve our quality? For some, the answer to the first part may seem easy, but to have a packaged improvement plan that includes both aspects appears to be more indefinable. So what can the biopharm industry do to counter these daily pressures? The answer may not be as elusive as you think. Companies need to drive innovation in new ways; in other words, they have to be innovative about innovation. Ways need to be found to drive innovation faster and with less expense, to sustain reductions in cost and continue to drive quality and operational excellence. Knowing what needs to be done, the question remains, how can it be done? The answer is actually fairly simple – a partner needs to be found that has as much to contribute and gain from the success of the organization itself. But who is the most obvious choice? The strategic suppliers to the organization! These are natural partners; your vendors know your processes and their products, and how to make your systems work most effectively. The main points to consider are as follows:
- Strategic suppliers often have as much to gain as the supplied organizations do — the main ingredient for a win–win relationship
- Most suppliers are eager to help and provide assessment or survey services to start the process
- Suppliers are the experts on their products and applications
- Supplier innovation is typically the least costly, risky and fastest way to market
- Supplier innovation occurs exponentially when compared with biopharm organizations’ innovation.
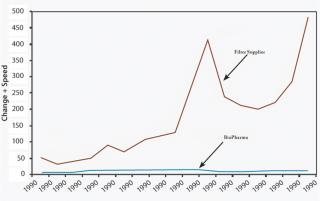
New applications or significant process developments can occur weekly with suppliers, whereas biopharm product innovations usually happen on an annual basis — at best — because of the lengthy and tedious development cycle. As most blockbuster drugs have a long lifecycle, several renditions of process innovation are necessary to continually reduce costs and drive revenue throughout the life of the drug. One biopharm drug can leverage thousands of products from a supplier’s portfolio, providing them with optimal solutions for the drug production process (Figure 1).
The How: Lay out a Roadmap
The key to success with such an optimization initiative is to lay out a plan with a clear roadmap. So many organizations discuss innovation in their strategies but rarely lay out a roadmap of how they will achieve this. The roadmap should provide a structure for the organization and working teams to follow, but be flexible enough for teams and suppliers to drive innovation in the way that best meets their project or application needs.
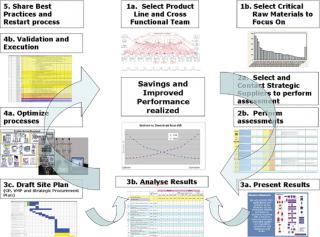
The Roadmap: Five Key Steps
When an organization determines that process innovation is an imperative, there are five key steps that can be followed to increase return and achieve the desired results (Figure 2).
Select what systems or product lines to focus on: Organizations should look at improvement initiatives from a strategic perspective: what product lines need to be leaned out the most, what areas or process steps are feeling the most pain, and where are the highest value losses. Tools such as Value Stream Maps and Site Gap Analysis may be good starting points to identify and prioritize which product lines or areas to start the optimization effort with. This approach is critical; it drives alignment with initiatives and ensures that innovation is focused in the right areas and doesn’t create bottlenecks.
Within the product line, determine the major/critical components and raw materials. The team should start by looking at the spread of raw materials, components and process steps across the site, looking at their
- largest areas of spend, either as cost of goods or yield loss
- largest inventory/volume areas
- potentially biggest problem areas and/or risks
- highest value generation.
Using simple tools such as Pareto Analysis, the team can narrow down and prioritize the efforts.
Select and Contact Strategic Suppliers for Site Surveys: Site surveys are a service that most suppliers provide free of charge to understand the process needs of their customers and to provide the best innovation to meet those needs. This is ideal, because it immediately provides a win–win situation. The customer gets an overview of the best supplies available for their processes and the supplier has an open audience to share their newest innovation with. This, of course, requires planning in advance. Usually, the top two or three suppliers in a category are selected and brought on-site to assess the entire manufacturing process. Additionally, planning a plant survey of the entire process requires absolute trust in the surveying vendor, as well as a willingness to be subjected to observations that could be perceived as critical. Seeing every process step and learning the processing parameters allows the supplier to provide recommendations for an optimal solution. Also, having multiple suppliers assess the process provides a comparison and an alternate source for risk mitigation. This approach also removes bias and allows the selected suppliers to perform the assessment with the same opportunity to review the process and propose solutions. Once the survey is complete, the suppliers should provide a formal report and presentation to highlight their solutions. The assessment (survey) and priority suggestions in the report allow the internal optimization team to prioritize efforts further and set timelines and milestones for each project.
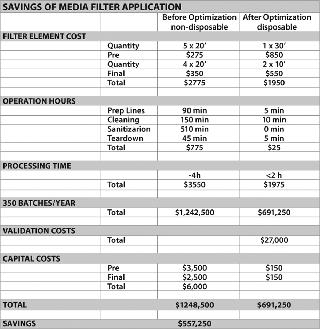
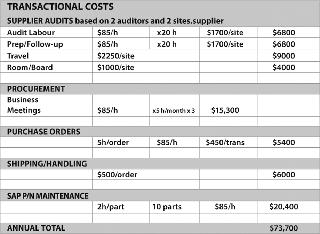
Process surveys allow suppliers to observe the total process and its critical unit operations, and provide a full solution rather than cookie cutter improvements that only optimize single process steps and provide sub-par results. Taking a holistic approach across the product line, with one lead team monitoring the portfolio, allows the team to look at the total opportunity from a total cost of ownership approach. Taking this approach allows all objectives to be achieved, including bottom line cost savings, cost avoidance or additional revenue creation (Figures 3 and 4).
Benefits that can be achieved include the following:
- >30% bottom line savings
- increased speed and efficiency — improved flow rates
- improved throughput
- reduced square footage
- reduced inventory levels
- reduced prep time
- improved operator safety and ergonomics
- reduced hold-up volumes
- improved yields — improved non-specific adsorption
- disposable applications
- reduced cleaning time
- higher capacity utilization.
Additional Benefits
Regulatory: Comparability studies can be drafted in advance to lay out the regulatory requirements upfront. This allows the portfolio manager to provide a more accurate cascading timeline with built in levels of regulatory communications.
Validation: Owing to the site-wide holistic approach, the entire filtration project on-site can be laid out in the site Validation Master Plan to ensure alignment and prioritization with other validation projects. Post-filing requirements and criticalities can be reviewed and put into an economical perspective. The monitoring and tracking can also be performed in a controlled system. Additional validation support can be mustered from the supplier service base.
Strategic Procurement: Cross-functional involvement from the Procurement Department is a must, allowing a supplier strategy to emerge and allowing for improved strategic planning and negotiations. Once each supplier brings their value to the table, the team can determine what the best strategic direction is (reducing supply base, driving volume with strategic suppliers, rewarding good performance, reducing transactional costs, etc.).
Supplier Relationships: Once an initial selection is made, the assessment allows each supplier to be judged on a level playing field. As the decision process is built in to decision matrixes and a team approach is taken, the overall supplier relationship can be improved and driven across the organization, ultimately strengthening the relationship with the strategic suppliers.
Risk Mitigation: This allows for side-by-side validation to be performed, allowing alternative sources to be validated in the same resource effort. Cost avoidance by leveraging supplier validation resources removes a large portion of the pain associated with validations. Cost avoidance depends on the application being assessed but, typically, filter validation can cost more than $25,000 in validation resources. Often, this can be reduced by more than 50% by leveraging supplier resources.
Present Business Case and Launch Site-Wide Projects
Each project milestone will have critical decision points, such as supplier selection and the assignation of business, testing and validation needs. It is critical to ensure that all cross-functional resources are available. Decision matrixes are a good tool to ensure the total cost of ownership approach is being taken and that personal bias is removed (Figure 5).
Value analysis must be reviewed to determine what solutions need to be implemented. Savings made on the cost of the filter may be passed up for savings on labour. For example, moving towards disposable applications typically increases the cost of filters by more than 30% — but can save significant labour hours/days because of reductions in cleaning time, prep time and teardown. Such savings may be preferred, compared with filter cost savings, because of objectives to reduce overheads or cycle times. Disposable systems also have the potential to reduce hold-up volumes, which can cause tremendous product value losses. The entire cost/benefit balance needs to be reviewed, as opposed to a single parameter, such as a meagre product discount.
Process Optimization Using Supplier Solutions
Once the projects have been identified, the detailed work will verify the observations of the survey report and identify the required solutions. Such work is best performed with the support of the supplier. The experience and competencies of the supplier should be utilized to implement possible solutions. Having a thorough report of what could be is nice — but execution is what actually provides the real results. Optimizing systems that have not been improved in the last 3 years typically yields greater than 30% savings or additional market value. The key to any improvement is driving true value into the network. This should be tracked to ensure that targets are being met and maintained.
The front-end value creation starts with bringing suppliers in for bench-top studies to confirm initial opportunities. This starts with small-scale (indicator) trials that are then scaled up to intermediate, pleated devices (verification trials) and, finally, large-scale runs (confirmation trials) and validation (Figure 6). Most suppliers, including filter suppliers, provide a range of total services to support small-and medium-scale filterability testing followed by full-scale validation.
Share Best Practices and Start the Improvement Cycle Again
Once proven results are achieved, it is critical to share them with other sites or product lines. Global organizations can leverage changes across the entire organization. Taking a more strategic approach allows organizations to focus at scale with suppliers that provide the most value, allowing the Purchasing Supplier Management organization to be more efficient and successful in reducing total spend — even with standard supplier pricing increases.
Conclusion
Supplier innovation is the fastest and least costly innovation that biopharm organizations can draw from. Using filtration as an example, it is apparent that significant changes in filtration technology during the past 10 years have steadily increased filtration capabilities and have rendered many filters obsolete. Any changes to systems that are more than 3 years old can provide significant savings and opportunities. Biopharm organizations can fill in resource gaps by leveraging suppliers’ total service packages. These costs are often already built in to the costs of the supplies, so are essentially wasted by manufacturers that choose not to utilize them. As seen in Figure 2, supplier innovations can be leveraged according to a standard process that can be further developed, based on the needs of the organization. Supplier innovation is truly an imperative in modern-day biopharm and should be taken into account during strategic planning and prioritization.
REFERENCES