Although significant scientific progress has been made in the biotechnology industry, it has lagged behind other sectors — such as aviation and automotive — in developing, scaling up, and industrializing products coming out of R&D. The goal is to implement robust and reliable manufacturing processes for good manufacturing practice (GMP) market supply cost-effectively. But manufacturing methods for biologics have remained unchanged for decades, with large-scale, capital-intensive stainless steel facilities taking three to five years to build and remaining both energy intensive and expensive to operate. When you take into the account a strong attrition rate through the different clinical phases for biologics and the fact that many drugs do not make it to market, that business model always represented high risk because such facilities were fixed, with little or no flexibility for redesign if a drug didn’t make it to market
Although significant scientific progress has been made in the biotechnology industry, it has lagged behind other sectors — such as aviation and automotive — in developing, scaling up, and industrializing products coming out of R&D. The goal is to implement robust and reliable manufacturing processes for good manufacturing practice (GMP) market supply cost-effectively. But manufacturing methods for biologics have remained unchanged for decades, with large-scale, capital-intensive stainless steel facilities taking three to five years to build and remaining both energy intensive and expensive to operate. When you take into the account a strong attrition rate through the different clinical phases for biologics and the fact that many drugs do not make it to market, that business model always represented high risk because such facilities were fixed, with little or no flexibility for redesign if a drug didn’t make it to market
Over time, the industry has come to realize that such a business model is no longer sustainable. Significantly increased titers, the end of the blockbuster era, and increasing cost pressures have led to adoption and use of smaller-scale equipment. If such equipment is available in single-use format, organizations can operate highly flexible multiproduct facilities that lower the barriers to entry to biopharmaceutical manufacturing for many companies — and countries — that could not previously afford such investments.
In addition, a phased implementation of single-use process equipment can help mitigate risk by delaying capital expenditure until the likelihood of product success is established. Such a radical change in the bioprocess development and manufacturing business model brings fresh challenges of its own; tried and tested methodologies must make way for radically new operating procedures.
Sartorius Stedim Biotech has experienced these changes first hand throughout its over 30 years in the biopharmaceutical project business — and coming from a traditional engineering background. The trend we are witnessing is that biopharmaceutical end users seek to respond to changing industry needs by working directly with individual suppliers. Users frequently contract in consultants or entire engineering teams to describe what they need for project implementation. They ask their procurement organizations (internal or external) to try to compare like with like among different technology suppliers that (because industry standards are still lacking) are each developing testing methodologies to qualify their own technologies. Those departments must execute their jobs and for the best (cheapest) prices. As part of the supplier and technology selection process, end users must audit different suppliers’ manufacturing, quality, and supply chains — again, without having defined industry standards as a benchmark against which to audit. Is this the way to ensure a robust, reliable, sustainable bioprocess platform for the future?
At our company we have learned and demonstrated that tried and tested methods can provide excellent bioprocess solutions through integrated solutions. We work with companies to guide them through the transition from traditional approaches toward new technologies for integrating project design and management.
Project Management and Implications of Single-Use
The goal today for bioprocess operations is to decouple process design from facility design. That can be achieved by maximizing the use of single-use systems, ideally through a fully single-use line but if not, by seeking a hybrid solution. Any bioprocess using significant numbers of single-use systems requires a radically different project management approach from that of a traditional engineering project. Such a radical approach runs both the facility design/build in parallel with process design, resulting in much shorter timelines compared with those of traditional projects.
Key components to this project management concept are, early in the project, to develop a design and business case for implementing single-use technologies using innovative software tools
- initiate supplier evaluation early in the project
- conduct single-use quality assessments and validation at the project start
- develop a layout, workflow, and facility design
- design a facility and process-automation strategy.
After a process is fully designed and associated equipment specified, we consider it important for a client to test and train staff on the actual equipment in our facilities before shipping to its own site.
Design and Business Case Analysis
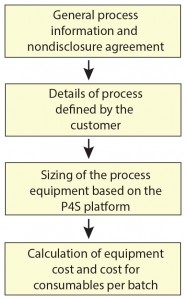
Figure 1: Turning process information into
cost and performance numbers to support the
decision-making process
The earlier our bioprocess consultants are involved in a project, the more opportunities will be open to revisit a process and define the right process technologies. Early phase 1 and phase 2 clinicals are ideal starting points because process and technology choices are yet to be locked down. Our bioprocess engineering consultants will analyze a process based on generic information that clients choose to share with us. We want to simplify interactions with our customers and be as precise as possible without making them feel obliged to disclose too much sensitive process information. The Sartorius “Process4Success” platform (Figure 1) enables multiscenario what if analyses of different process and technology options. Along with our own simulation tools, we use the BioSolve process modeling tool from Biopharm Services to define which mix of technologies for a given process at a given scale and titer will provide the highest overall performance and lowest cost of goods (COGS, Figure 2).
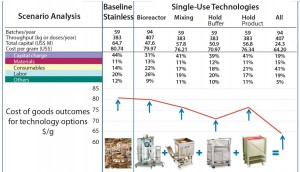
Figure 2: Multiscenario analysis for process and technology selection using the BioSolve process
modeling software
The modeling and simulation tools are an excellent aid to decision making regarding when and where it makes sense to work with single-use technologies and where a hybrid approach may offer greater value. To enable clients to anticipate possible scale-up issues, such tools can simulate what a process currently at pilot scale will look like when it is scaled up and brought to commercial manufacturing. We provide a detailed report and a first budget figure for investment and operating costs, depending on the number of batches per year. It can be used as an aid to decision making about investments that need to be made now and which investments can be delayed through staged implementation of single-use technologies.
If a design already exists, it is often possible to use the same process modeling and simulation tools to optimize process performance and reduce both operating and capital expenses (OpEx and CapEx costs). This approach can even be applied to existing stainless steel processes that users decide to revisit in hopes of improving COGs and productivity by implementing new process technologies.
Evaluating Suppliers, Systems, and Supply Chains
We encourage clients to spend time at our facilities that supply hardware, software, and consumables as early on as possible in the project. When you move to a single-use or hybrid process train, you need to have an in-depth understanding and feel fully confident about the manufacturing, quality, and supply-chain stand ards under which your supplier is operating. You need to develop a level of relationship that is more than that of a customer with a vendor, indeed more than even a close collaboration: This relationship serves as an extension of your own organization. We can guide companies that are implementing single-use systems for the first time through relevant regulatory references (where available). If such references are not available, we will explain the standard test methods we are working with and the rationale for their development and implementation. We invite conversations with other clients who have successfully implemented fully single-use and hybrid process trains in CGMP pilot and commercial facilities worldwide.
Carry Out Single-Use Quality Assessments at Project Start
It is important to perform a risk assessment of materials used to manufacture single-use systems for your bioprocess. Because it has the longest contact time with the fluid, single-use bag film is the most critical component. For example, Flexsafe polyethylene film is fully scalable from 50 mL to 2,000 L and was developed to address the issue of poor or inconsistent cell growth in single-use bags. A standardized cell growth assay was used to optimize the film formulation; define the operating ranges for extrusion, welding, and gamma irradiation processes; and to establish specifications and process controls (Figure 3).
Our project managers help clients evaluate the extractables data package for film and components. An extrapolation evaluation then can assess the possible quantity of extractables in an intermediate active pharmaceutical ingredient (API) or drug product (all extractables could be leachables), which can then be submitted for toxicological assessment. If leachables are a concern, a test should be performed under real contact conditions, including process risk analysis and whatever leachables testing is identified as necessary.
Develop the Layout, Workflow, and Facility Design
Facility Layout: In addition to optimizing process design, we can help our clients design a facility layout based on equipment selection. Working with single-use closed systems enables downsizing of higher classification cleanroom areas.Large-volume support solutions can be stationed in a controlled non-classified (CNC) environment and fed through the wall using a transfer hatch into higher classification areas. With a single-use–based design, ISO8 and ISO7 cleanroom surfaces can be reduced by an average of 20%. Moreover, overall cleanroom volumes can be decreased by 26% by lowering the cleanroom ceiling heights usually required for stainless steel bioreactors. HVAC systems also can be downsized by an average of 33% regarding air supply requirements, although that does vary from project to project (1).
Workflows: Whether a production facility is a retrofit or greenfield project, the process and workflows must be defined to develop the facility layout. Furthermore, logistics must be fully specified for material handling, critical step segregation, work-in-progress space, and storage requirements, as well as the utilities required for process and cleaning needs at every critical process step. Finally, required support spaces must be clearly defined, including their interactions with the process path.
The logistics and handling of single-use systems are, of course, very different from operations in hard-piped facilities. For that, ergonomics and workflow require careful consideration. For bag-based transport and for storage of raw materials and semifinished and finished products, horizontally positioned production operations located on one floor, with sufficient space for material and personnel transfer, represent the most suitable layout for single-use production facilities. Single-use process lines offer the chance to simplify material transfer, circulation space, and material management and downsize both water and utility requirements (70–80%), enabling end users to implement a truly sustainable facility (Figure 4).
Facility and Process Automation Strategy
Besides bioprocess equipment, automation and integration into a given IT infrastructure is key for successful project execution. Because no single standard exists for the pharmaceutical industry, and many customers are using a number of components (sensors, actuators, local controllers, DCS, SCADA, and so on) from different vendors, our company decided to strictly follow the GAMP principles of the V-model (2). Once user requirements are clearly defined, our automation engineers translate them into functional specifications and afterward into a hardware and software design specification, followed by a design qualification phase. In parallel we offer different options in automation architecture. It can be based on our proprietary solutions (DCU, MFCS) or on third-party, industrial solutions (Siemens, Emerson, Rockwell) or even a mixture where appropriate. The goal is flexibility in automation and for companies to find the most suitable solutions for their requirements. We can create/build a solution and carry out the respective test work accordingly.
Training and Application Support
As the integrated approach proceeds, a project manager will call on specific applications specialists to help train operators. This is particularly important for single-use systems with which end-user operators may be less familiar. Application specialists typically conduct training at a client’s facility. Our single-use training options include theoretical on-site and e-learning options available at Sartorius College and hands-on training at our applications centers in Göttingen (Germany), New York (USA), or Shanghai (China) — wherever it is practical for a given client. We can support our customers in ramping up production processes as well as becoming familiar with new process equipment.
Project Communication
Those of you who are experienced in biopharmaceutical project execution are aware of the milestones that need to be reached.
After the concept phase comes the Approved for Design milestone. Upon completion of basic engineering, the customer will sign the Approval for Construction — Sartorius starts the production/assembling phase of the systems (hardware/software and single-use consumables). The IQ/OQ as well as the PQ (installation, operational, and performance qualifications) are of utmost importance for our customers to prove the right functionality and performance.
The test plan is defined jointly by our customers and by Sartorius and leads to the milestone, Approval for FAT (factory acceptance test). This initiates the execution in our factory by our qualification team. The customer can witness these tests if appropriate or required. To be fast and efficient, we always try to do as much test work as possible on our premises so as to prevent carrying out testing twice. All remaining tests or those that require special utilities (that as those available only on-site) will be performed after shipment and commissioning.
To ensure availability and readiness for on-site activities, we have integrated the milestone Approved for Shipment. This will help us synchronize with other activities of our customers and/or third party contractors. Once the equipment is shipped, installed, and finally tested, the customer will sign the acceptance protocol. This will be the milestone Customer Acceptance for the process equipment.
Case Study: WuXi App tecEstablished in December 2000, WuXi Apptec Biopharmaceutical Co. is a leading global WuXi involved Sartorius Stedim Biotech in early discussions regarding process design As a result, this project was completed on schedule (within an 18-month timeframe) |
Significantly Reducing Timelines
By following the project management methodology for single-use and hybrid process lines outlined herein, companies can significantly reduce costs and shorten timelines relative to traditional project delivery models.
The bioprocess industry is evolving, but there is still a great deal of trial and error regarding implementation of next-generation bioprocess platforms, with many people learning “on the job.” SSB is actively engaged in industry working groups toward a goal of establishing more industry consensus standards. Those applicable for both bioprocess end-users and suppliers will need to receive endorsement by regulatory authorities as we all seek to ensure the rapid delivery of robust, reliable bioprocesses that meet the sector’s future bioproduction requirements. •
References
1 Sinclair A, et al. A Sustainable Single- Use Facility for Monoclonal Antibody Production. Bioprocess Int. 11 (11) 2013: 34–40.
2 GAMP 5: A Risk-Based Approach to Compliant GxP Computerized Systems. ISPE, 2008; www.ispe.org/gamp-5.
Corresponding author Thorsten Peuker is vice president, integrated solutions (Thorsten.Peuker@sartorius.com) and BPI editorial advisor Miriam Monge is director of marketing, integrated solutions (miriam.monge@sartorius.com).
For more information, please visit the BPI website and Sartorius’s special issue from September 2014, www.bioprocessintl.com/category/2014/ september-2014-sartorius-supplement.