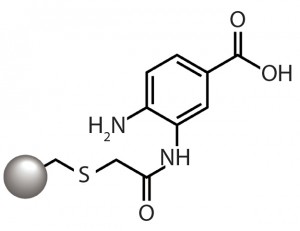
Figure 1: The aminobenzoic acid ligand of
the CMM HyperCel mixed-mode cation
exchanger provides cation-exchange
functionality through the carboxyl group and
hydrophobicity-based binding through the
aromatic ring.
The biopharmaceutical industry is under a great deal of pressure to modernize manufacturing to meet the challenges of production at vastly different scales for niche drugs as well as for expected massive blockbusters, biosimilars, and regional manufacturing. To address these challenges, the biopharmaceutical industry is embracing process intensification through single-use and continuous processing technologies. Implementing these technologies offers increased productivity and manufacturing flexibility and reduces the footprint, capital outlay, and operating costs.
Pall Life Sciences has developed several technologies designed to enable single-use and continuous processing for a broad range of downstream unit operations. The Pall systems are designed to be interconnected to offer not just increased productivity at a single step, but also an overall improvement in process economics and throughput and a significant reduction in overall process time.
To demonstrate the potential benefits of combining continuous operations for monoclonal antibody (mAb) purification, three continuous chromatography steps were linked together to improve productivity and reduce the overall time to process, while still achieving stringent quality targets.
Read the full text of this article in the PDF (Login required).
