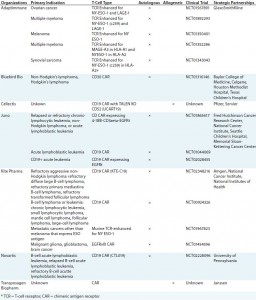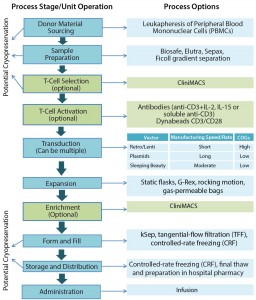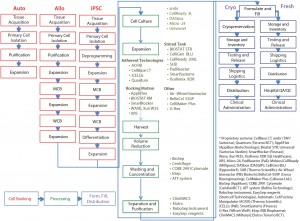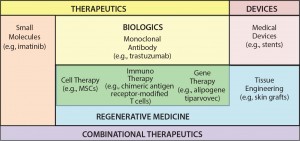
Figure 1: The 21st-century pharmacopeia, including the convergence of “regenerative medicine” therapeutic approaches
Cell-based immunotherapies (iTx) are emerging as a truly transformative therapeutic modality that is both complementary and convergent with existing regenerative medicine approaches, including gene therapy, cell therapy, and tissue engineering (Figure 1). Critically, iTx offer step-change improvements in efficacy compared with current standards of care (1) for a range of clinical indications and unmet therapeutic needs — particularly oncology.
The clear efficacy of iTx is in contrast with some previous regenerative medicine approaches, including early mesenchymal stem cell (MSC) therapies for articular chrondral repair, which demonstrated only equivocal efficacy (2) despite a substantial increase in cost as compared with existing standards of care (3). Therefore, although clinical adoption (4) and pharmaceutical engagement in regenerative medicine may have been limited to date (5, 6), the “apparent curative potential” (7) of iTx has driven significant early-stage investment from pharmaceutical companies (Table 1), including those not previously publicly engaged in regenerative medicine. However, as history has proven, the complexity of these therapies often means that clinical efficacy is not a guarantee of commercial success (see “Provenge” box).
| Provenge (Sipuleucel-T) in Retrospect “Overhyped” initial commercialization plan Cumbersome administration Controversial (proposed) mechanism of action (MoA) Minimal reported efficacy (few prostate specific antigen declines) No measurable prolongation in time to first progression (TTP) Cost and initial uncertainty of reimbursement High cost of goods (CoGs) (Reference: personal communication with P. Kantoff ) |
Cell-based immunotherapies pose a number of unique bioprocessing challenges, in particular those pertaining to the “autologous or allogeneic” decision (8). Historically, a debate has been sustained by both trade and academic literate (along with international conferences) based on the assumption that it is a simple engineering choice. Fundamentally — in almost all cases — that decision is perverse because bioprocessing requirements are dependent on characteristics of individual therapeutic agents, with “autologous or allogeneic” representing an unavoidable immunological feature (9).
The same is true for iTx, with the majority of those in late-stage development being autologous (10, 11).
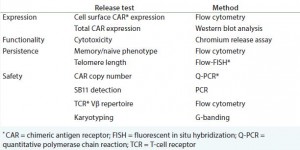
Applying what the industry has learned from biomanufacturing previous autologous regenerative medicines (12, 13) (see the “Provenge” box), quality assurance (QA) testing — including patient-specific lot release — will be a major driver of cost of goods (CoG) and a critical source of process risk for iTx. Therefore, the field must further investigate and determine what steps the industry must take to move toward a robust, systematic, and pragmatic process for the release testing of autologous cell‑based immunotherapies, while keeping in mind both time and cost.
Major Bottlenecks in iTx Biomanufacturing
Autologous immunotherapy manufacturing bioprocesses (Figure 2) are inherently more complex in terms of the number of unit operations, the sensitivity of process raw materials to the biomanufacturing environment, and the complexity of available unit operations as compared to MSC manufacturing sequences (Figure 3) (14, 15). For example, a typical iTx work flow may be best considered as two separate work flows: the bioprocessing and manipulation of patient-specific material and the generation of vectors to transduce donor samples, conferring efficacy. Thus, the bioprocessing community is faced with two key iTx process bottlenecks: scale-up and QA/QC.
iTx Process Scale-Up
Donor Material Variability: A range of factors contribute to variability of patient-specific samples as the principal raw material in iTx processing. They include variability of clinical acquisition as it pertains to the skill and/or work practice of individual clinicians; extent of patient disease progression; patient medication regimes; and distribution procedures. Additionally, preparation protocols for products in early stage trials using a Ficoll gradient are subject to operator variability.
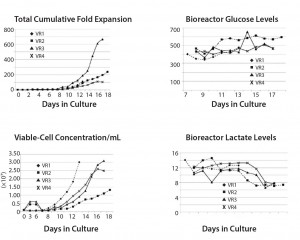
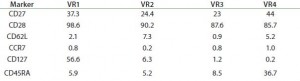
Transduction Efficiencies: The scalability of vectors — usually using a stirred tank reactor (STR) — and the sterility and specificity of their purification represent a critical bottleneck in iTx production. For example, Hollyman et al. (14) reported modest but statistically significant variability in transduction of clinical-grade autologous T cells. Those cells were derived from patients with chronic lympoblastic leukaemia (CLL), genetically modified with a replication-defective gamma-retroviral vector encoding a chimeric antigen receptor (CAR) targeted to CD19 (1928z) (Figure 4). Such variability is similar across all cell types and vectors, contributing to uncertainty in CoGs and a need for robust QA/QC to ensure interdonor process consistency to regulators.
Impact of Variability on Cell Expansion and Critical Quality Attributes (CQAs): Multiple sources of process variability culminate in variability in cell expansion and CQAs, thereby contributing to challenges in bioprocess control and reproducibility. Because of such heterogeneity, manufacturers operating without robust in-line monitoring tools (for cell counting, glucose, lactate, pH, and dissolved oxygen) may find it difficult to determine the relationships among specific sources of variability that can be controlled in initial unit operations as well as their cumulative impact on final therapeutic products. The real need to ensure that systems for in-line process monitoring, control, and data visualization are in place is a greater priority than optimizing processes from a conventional CoG perspective (such as extent of expansion). That requires monitoring multiple dynamic factors at a frequency that facilitates acceptable product profiles and process control, which automates process actuators to maintain critical process parameters (CPP) within a process design space. Data visualization also is required. It should overlay process data collected in real-time with previous acceptable iTx manufacturing runs and within predetermined process tolerances.
In other words, it is essential for manufacturers to “reverse engineer” from product CQAs agreed with regulators and ascertain what proxies (e.g., in-line pH, dissolved oxygen, glucose and lactate) vary throughout the process. Manufacturers also should determine ranges that are acceptable and/or require change in process conditions to ensure maximal adherence to acceptable product profiles for a commercially viable combination of process time and cost. Such a multivariate “golden batch” approach can permit real-time release testing. But it is subject to the provision of robust and validated process data for a range of donors and technology sources (to use clinical trial nomenclature, a suitable power number of patients and processes).
Advanced Online Process Analytical Technology (PAT) Compared with Manual Sampling: Each time an operator removes a sample, the potential exists to introduce contamination that can lead to patient-treatment failure. Integrated sensor technologies that do not compromise a vessel’s sterile containment innately minimize this risk. Further, resulting sensor data — if logged automatically to a 21 CFR part 11–compliant supervisory control and data acquisition (SCADA) software — serves to generate an electronic batch record that can be used as a continuous QC document and regulatory audit trail.
One integrated technology that is already accepted by the market and in regular use is pH and DO fluorometric sensors from PreSens Presicion Sensing GmbH. A novel and emerging option is technology from Aber Instruments Ltd. that measures viability based on radio-frequency impedance. Available as single-use probes integrated into rocking motion bags, Aber sensors consist of high-density polyethylene (HDPE) with platinum electrodes that meet USP and Class VI requirements. As opposed to taking daily samples of a given culture to assess its viability, continuous monitoring of cell expansion allows determination of optimal harvest point. That eliminates the repeated daily risk of potential contamination.
Minimizing the potential human‑operator impact of a highly heterogeneous process and automating control of parameters (where possible in a system) both have clear benefits. Each batch should be maintained within a prescribed design space based on historically informed ranges, through online feedback control to tightly align a process within a known biomanufacturing trajectory. That will enable better interdonor process consistency and potentially reduce the amount of required release testing. Tangible outcomes of such highly controlled processes could be higher therapeutic product quality and reduced CoGs — benefitting manufacturers, regulators, and most importantly, patients.
As iTx progress toward commercialization, striving for real-time release testing (RTRT) is important and achievable (see sideboxes “Benefits of Implementing RTRT” and “Critical Challenges”). The requisite investment required to sufficiently characterize process cell lines and the provision of process analytical tools that do not compromise sterility, are worthwhile and will require multistakeholder engagement to specify and implement. The result of such efforts will be greater process robustness, lower CoGs, and broader patient access to this extraordinary therapeutic modality.

Table 4: Comparison of offline and online analyzers (adapted from Sartorius “Pop-up parameter table”)
| Minimum Quality Threshold Parameters for Dendritic-Cell Vaccines | ||
| General MQTs to Be Tested • Cytokine production/lytic activity when incubated with target cells • Phenotypic characteristics (using fluorescence-activated cell sorting) • Correlation of potency with transduction efficiency (% transduced cells/vector copy number per cell) • Testing for replication-competent vector culture methods (expensive, time consuming) or PCR (faster and cheaper, but has risk of false positive results and requires long-term (15 year) follow-up) • Antigen-specific CD4+ and CD8+ T-cell counts in peripheral blood |
• Phenotypic analysis, including tetramer analysis • Functional analysis (ELISpot, cytokine production and/or cytotoxicity) • Purity • Morphology • Phenotype analysis • Viability • Phenotypic stability • Immune response induction • Analysis of T-cell receptor repertoire • Sterility (bacteria and fungi) • Mycoplasma |
• Visual inspection • Endotoxin LAL • Screen for unwanted autonomous growth Trial/Patient-Specific MQTs • Presence of antigen-specific T-cell • Antibody titers (if relevant) • Antigen expression on tumor cells • Antigen-specific antibody titers in serum • Immunohistochemistry (e.g., measurement of antigen loss variants) |
Acknowledgments
The authors are grateful for the personal feedback and guidance offered by Mark Angelino (Bluebird Bio) and Knut Niss (Novartis) in the writing of this piece as well as the following companies and organizations that have contributed to the Centre for the Advancement of Sustainable Medical Innovation (CASMI) Translational Stem Cell Consortium (CTSCC) as funding and events partners, without whom the consortium and the benefits it will bring to stem-cell translation would be constrained: GE Healthcare, Center for Commercialization of Regenerative Medicine (CCRM), Sartorius Stedim Biotech, Lonza, the California Institute for Regenerative Medicine (CIRM), the Strategies for Engineered Negligible Senescence (SENS) Research Foundation, UK Cell Therapy Catapult, the US NIH Center for Regenerative Medicine, the New York Stem Cell Foundation (NYSCF), ThermoFisher Scientific, Eisai, Medipost (US), Medipost (Korea), Celgene, Roche, and Oxford Biomedica. Author David A Brindley gratefully acknowledges the support of Sue Dopson (SBS), Andrew Carr (NDORMS), and Felix Reed-Tsochas (SBS) as well as personal funding from the Oxford Musculoskeletal National Institute for Health Research (NIHR) biomedical research unit, the Saïd Foundation, and the SENS Research Foundation.
Disclosure
This publication represents the individual opinions of the authors and may not necessarily represent the viewpoints of their employers. Author Brindley is a stockholder in Translation Ventures Ltd., a company that among other services provides cell therapy biomanufacturing, regulatory, and financial advice to clients in the cell therapy sector. He is subject to the Chartered Financial Analyst (CFA) Institute’s and Chartered Alternative Investment Analyst’s (CAIA) codes, standards, and guidelines, so he must stress that this article is provided for academic interest only and must not be construed in any way as an investment recommendation. Additionally, at time of publication, Brindley and the organizations with which he is affiliated may or may not have agreed-upon and/or pending funding commitments from the organizations named herein. Authors Bure, Tindal and Fuerstenau-Sharp are employees of Sartorius Stedim Biotech.
References
1 Kalos M, et al. 2011. T Cells with Chimeric Antigen Receptors Have Potent Antitumor Effects and Can Establish Memory in Patients with Advanced Leukemia. Sci. Transl. Med. 3 2011: 95ra73.
2 Brittberg M, et al. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 331, 1994: 889–995.
3 Samuelson EM, Brown DE. 2012. Cost-Effectiveness Analysis of Autologous Chondrocyte Implantation: A Comparison of Periosteal Patch versus Type I/III Collagen Membrane. Am. J. Sports Med. 40, 2012: 1252–1258.
4 Davies BM, et al. Quantitative Assessment of Barriers to the Clinical Development and Adoption of Cellular Therapies: A Pilot Study. J. Tissue Eng. 5, 2014: 2041731414551764.
5 Brindley DA, et al. The Implementation of Novel Collaborative Structures for the Identification and Resolution of Barriers to Pluripotent Stem Cell Translation. Stem Cells Dev. 22 Suppl 1, 2013: 63–72.
6 Mullard A. Advancing Regenerative Medicine. Nat. Med. 20, 2014: 795.
7 Flemming A. Deal Watch: Pfizer and GSK Join Race for T Cell Cancer Therapies. Nat. Rev. Drug Discov. 13, 2014: 568–569.
8 Kirouac DC and Zandstra PW. The Systematic Production of Cells for Cell Therapies. Cell Stem Cell 3, 2008: 369–381.
9 Fairchild PJ. The Challenge of Immunogenicity in the Quest for Induced Pluripotency. Nat. Rev. Immunol. 10, 2010: 868–875.
10 Rosenberg SA. Adoptive Immunotherapy of Cancer: Accomplishments and Prospects. Cancer Treat Rep. 68, 1984: 233–255.
11 Fox BA, et al. Defining the Critical Hurdles in Cancer Immunotherapy. J. Transl. Med. 9, 2011: 214.
12 Monesmith TT. Meeting the Challenges in Manufacturing Autologous Cellular Therapies. Bioprocess Int. 2011 9(3) 2011: S38–S41.
13 Ratcliffe E, Thomas RJ, Williams DJ. Current Understanding and Challenges in Bioprocessing of Stem Cell–Based Therapies for Regenerative Medicine. Br. Med. Bull. 100, 2011: 137–155.
14 Hollyman D, et al. Manufacturing Validation of Biologically Functional T Cells Targeted to CD19 Antigen for Autologous Adoptive Cell Therapy. J. Immunother. 32, 2009: 169–180.
15 Kaiser AD, et al. Towards a Commercial Process for the Manufacture of Genetically Modified T Cells for Therapy. Cancer Gene Ther. 23 January 2015; www. nature.com/cgt/journal/vaop/ncurrent/full/ cgt201478a.html.
16 DA Brindley, et al. Cell Therapy Bioprocessing Technologies and Indicators of Technological Convergence. BioProcess Intl. 12(3) 2014: S12–S17. •
Corresponding author David A. Brindley is the Cooksey–Botnar–Saïd fellow in healthcare translation at the University of Oxford’s NDORMS and the Saïd Business School, research fellow at the Harvard Stem Cell Institute, senior research fellow at the University College London’s Centre for Behavioural Medicine, a member of the Stanford–UCSF FDA Centre for Regulatory Science and Innovation and the corresponding author (david.brindley@ ndorms.ox.ac.uk). Rahul Rekhi is an advisor to the chief medical officer of the UK Department of Health. Maya Fuerstenau- Sharp is the manager of R&D for Cell Culture Technologies at Sartorius Stedim Biotech. Philip W. Kantoff is the Jerome and Nancy Kohlberg professor of medicine at Harvard Medical School, director of the Lank Center for Genitourinary Oncology at Dana-Farber Cancer Institute and a physician at Dana- Farber Cancer Institute and Brigham And Women’s Hospital. Georg A. Hollander is a research professor of pediatrics at the University of Oxford. James A. Smith is a researcher at the Oxford-UCL Centre for Advancement of Sustainable Innovation’s (CASMI) Translational Stem Cell Consortium (CTSCC). Stuart Tindal is the PAT product manager at Sartorius Stedim Biotech. Nick Timmins is the director of product and process development at the Center for Commercialization of Regenerative Medicine (CCRM) Kim Bure is the director of regenerative medicine at Sartorius Stedim Biotech.