Mycoplasma are infamous for contaminating cell culture lines at rates as high as 80% (1,2,3,4,5). For biopharmaceutical processes, the inadvertent use of contaminated culture medium or medium components can lead to contamination of an aseptic process-validation media fill or cell culture medium for a bioreactor (6,7,8,9,10,11).
Thoroughly testing medium components before use is generally impractical because of the large volume of material in use. Frequently, culture media cannot be autoclaved (because of the presence of heat-sensitive components or large volumes), so sterilization by filtration is a practical alternative. Removal of mycoplasma with high assurance requires 0.1-µm rated filters. Currently, there is no standard method (no standard testing conditions) for rating such filters at 0.1 µm, and routine manufacturing tests are not always performed at elevated pressures. That has led to concerns regarding filter performance at elevated pressures.
PRODUCT FOCUS: ALL BIOLOGICALS
PROCESS FOCUS: DOWNSTREAM PROCESSING
WHO SHOULD READ: PRODUCT AND PRODUCTION DEVELOPMENT, CELL CULTURE ENGINEERS
KEYWORDS: CONTAMINATION, FILTRATION, PURIFICATION, VIRAL RETENTION
LEVEl: INTERMEDIATE
Our study aims to determine the titer reduction provided by Pall Fluorodyne EX grade EDT filter medium when subjected to bacterial challenges at differential pressures of 30 and 45 psid with a highly penetrative mycoplasma culture at a high challenge level. Filters were thus presented with a robust challenge, easily exceeding the generally expected exposure level.
In previous studies, we showed that although the maximum cell size of Acholeplasma laidlawii changed with nutritional conditions, the minimum cell size remained virtually unchanged, so all tested nutritional conditions resulted in a population of cells smaller than 0.2 µm (12). Further, cultivation in tryptic soy broth (TSB) resulted in an apparent increase in the percentage of very small cells. But those cells were actually less penetrative than cells cultured in other conditions. Ultimately, cultivation of A. laidlawii in growth medium supplemented with 10% horse serum provided the most penetrative (not necessarily the smallest) cells, which we used in our study.
Filters designed for mycoplasma removal/retention are generally challenged with mycoplasma cells at a minimum concentration of 1 × 107 colony-forming units (cfu)/cm2 of filter surface area. That is comparable to the ASTM-F838-05 standard recommendation for bacterial challenge of 0.2-micro;m rated “sterilizing-grade” filters, although some manufacturers may use less. In this case, we challenged with the above minimum cell concentration at 30 and 45 psid. We then exceeded that minimum by one log to evaluate the filters under the extreme challenge conditions of high pressure (45 psid) and high mycoplasma load.
Methods and Materials
We cultured A. laidlawii (ATCC 23206) in mycoplasma broth from frozen stock. The broth consisted of mycoplasma broth base (20 g/L), yeast extract (25 g/L), and 100 mL/L of heat inactivated horse serum. We incubated the broth culture at 37 °C for three days. A. laidlawii titer was determined using membrane filtration of the appropriate dilutions and plated on mycoplasma agar. Mycoplasma agar consisted of the same broth as described above, with the addition of 14 g/L agar and 13 mg/L crystal violet (to aid in visualization of the colonies). We inclubated the plates for 14 days at 37 °C. The dilution buffer consisted of 20 g/L mycoplasma broth base in deionized (DI) water.
The A. laidlawii culture fluid was subjected to cavitation in an ultrasonic cleaning bath for two to five minutes before use to decrease cell aggregation and then added to the 1 L challenge broth. We removed a sample of the challenge fluid to determine the actual viable concentration of mycoplasma cells.
We tested nine 47-mm disks of Pall’s Fluorodyne EX–grade EDT filter media (P/N EDT04725, with an effective filter area, EFA, of 13.8 cm2 from three separate manufacturing lots) at each test pressure and under each test condition. A positive-control filter of Pall’s Ultipor Nylon 6,6 (P/N FTKNR) was included to ensure mycoplasma penetration through an integral 0.2-µm sterilizing filter. We detemined average flow rate on the basis of the time required to collect the effluent volume (1 L). After the bacterial challenge test, we passed the entire filter effluent through a 0.1-µm–rated Nylon 6,6 analysis disk (Pall Ultipor, P/N NT09025), which was plated on mycoplasma agar (described above), incubated for 14 days at 37 °C, and examined for mycoplasma growth. The titer reduction (TR) for each filter was determined as follows: TR = (total number of mycoplasma influent to the filter) Ă· (number of colonies recorded on the downstream analysis disk).
When we detected no colonies downstream of the challenged filter disk, the titer reduction was expressed as greater than the total number of organisms influent to the filter, representing the minimum detectable titer reduction (given the influent concentration).
Results and Discussion
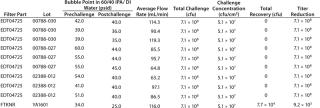
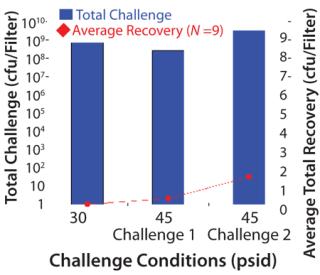
Table 1–Tables 2 and 3 chart the results of our mycoplasma bacterial challenges. The total challenge at 30 psid was 7.1 × 108 cfu/filter disk, resulting in a challenge of 5.1 × 107 cfucm2 of effective filter area (which is above the minimum target of 1 × 107 cfu/cm2). We detected no penetration at 30 psid, and the resulting minimum titer reduction was >7.1 × 108.
Table 1: Results of the first set of A. laidlawii filter challenge tests at 30 psid; the test filters (P/N EDT04725) were 0.1-µm rated filters, and the positive control filter (P/NFTKNR) was a 0.2-µm rated filter. The positive control filter was used to demonstrate the penetrative ability of the A. laidlawii cells used in the test.
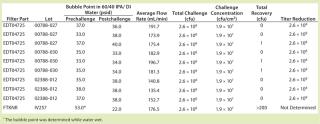
Table 2: Results of the first set of A. laidlawii filter challenge tests at 45 psid; the test filters (P/N EDT04725) were 0.1-µm rated filters and the positive control filter (P/NFTKNR) was a 0.2-µm rated filter. The positive control filter was used to demonstrate the penetrative ability of the A. laidlawii cells used in the test.
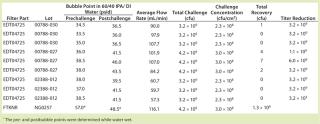
Table 3: Results of the second set of A. laidlawii filter challenge tests at 45 psid; in this test, the challenge concentration (cfu/cm2) was one log higher than the minimum required. The test filters (P/N EDT04725) were 0.1-µm rated filters, and the positive control filter (P/NFTKNR) was a 0.2-µm rated filter, which was used to demonstrate the penetrative ability of the A. laidlawii cells used in the test.
The first trial at 45 psid had a total challenge of 2.6 Ă— 108 cfu/filter disk, which resulted in a challenge of 1.9 Ă— 107 cfu/cm2 EFA. Six out of nine filters showed no penetration, and three were penetrated by a single cfu. In the second trial at 45 psid with higher challenge level, the total challenge was 3.2 Ă— 109 to 4.2 Ă— 109 cfu/filter disk. That resulted in a challenge of 2.3 Ă— 108 to 3.0 Ă— 108 cfu/cm2 EFA. We detected no penetration in five out of the nine filter disks tested at 45 psid with this elevated challenge level, and only one to seven cfu (out of a total 3.2 Ă— 109 cfu) penetrated the remaining filter disks challenged at 45 psid.
Our results show that the risk of penetration increases with increasing pressure and bacterial load. But using a low process pressure (≤=30 psid) and the presence of a low bioburden in the process fluid (which is much more realistic than the artificially high challenge levels used here) significantly reduces the likelihood of penetration by A. laidlawii through the Pall Fluorodyne EX grade EDT filter membrane.
Author Details
Martha Folmsbee is senior staff scientist, and Michael Moussourakis is market manager at Pall Corporation, 25 Harbor Park D., Port Washington, NY 11050; Martha_Folmsbee@pall.com. Pall, the Pall logo, Fluorodyne, and Ultipor are trademarks of Pall Corporation.
REFERENCES