Integrity testing of sterilizing-grade filters is necessary to reliably prevent damage to these sterile barriers from compromising the production of biopharmaceuticals. Documented integrity test results are essential to a manufacturing audit trail for releasing pharmaceutical products (1, 2). Accordingly, problems encountered during this testing can lead to considerable financial damages and substantial delays or even entirely prevent a production lot from being released to the market. Therefore, filter integrity testing is a critical step with high economic importance for biopharmaceutical companies.
Daily operations show time and again, however, that unnecessary problems can occur during and after integrity testing due to use errors and failure to comply with necessary basic conditions of the process. Essentially avoidable errors in data recording also can occur. Here I describe some measures that can significantly increase the reliability of filter integrity testing.
PRODUCT FOCUS: ALL BIOLOGICS
PROCESS FOCUS: Downstream processing and fill–finish
WHO SHOULD READ: PROCESS DEVELOPMENT, QA/QC, ANALYTICAL
KEYWORDS: FILTRATION, MEMBRANES, INTEGRITY TESTING, BUBBLE POINT, AUTOMATION, VALIDATION
LEVEL: BASIC
Background
Far-reaching consequences come when filters fail integrity tests. Ultimately, a suitable strategy must be used to determine whether a filter element is actually defective or the result was a false negative. In the latter case, the element would be evaluated as defective even though it is completely intact. The reasons for false-negative test results are numerous, and careful analysis is required to determine their real causes (e.g., system leakage, insufficient membrane wetting, and/or temperature effects). A PDA technical report on sterilizing liquid filtration excellently describes a systematic approach for analyzing such system failures (3), so this article focuses on the other factors that may not be so apparent but can cause problems in daily routine operations.
Automatic Recognition of an Unconnected Filter
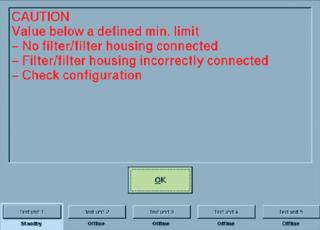
Self-locking, quick-connect couplings are typically used to secure the tubing of an integrity tester to the filter cartridge housing to be tested. But if a coupling is incorrectly fastened to that housing — or if the coupling is defective — then it cannot open correctly. Such a problem is not always immediately detectable from the outside. If a filter test begins in such a situation, the poorly connected filter element will not be tested because of the closed coupling. Instead, the integrity of the closed tubing will be tested. If a diffusion test is started, for example, no diffusion will take place. Because the maximum diffusion rate is the criterion for evaluating whether a filter passes or fails its integrity test, in this case a pass evaluation may be displayed. Experienced users immediately notice that an unrealistic diffusion rate of 0 mL/min indicates an error in the system. But less experienced users may not recognize such an error — or someone may be too busy performing daily routine tasks to notice. Similar problems can also occur when bubble-point tests or water-intrusion tests are performed.
The real dramatic aspect of those scenarios is that a test can be passed even if a defective filter is tested (false-positive result). There have already been incidents in which such errors were not noticed until an audit by regulatory inspectors. In such cases, product lots manufactured up to that point should not have been released. If a defective sterilizing-grade filter goes undetected in use, it can lead to unforeseeable consequences.
Plant operators thus should always ensure that their integrity testers can automatically detect such system errors and alerting users with error messages or warnings. Advanced integrity-test systems enable users to input specific minimum values for parameters such as diffusion, bubble-point test flow rate, and net volume — in addition to filter limit values defined by cartridge manufacturers, which include maximum diffusion and minimum bubble point. As soon as a filter element is connected to such a tester with tubing, a diffusion rate or net volume must be measurable. Zero values (or those approximating zero) indicate a system error, as explained above (Figure 1).
False-positive test results can have far-reaching consequences. But the following (basically avoidable) problems can occur during test sequences or after an integrity tester has been used for many years.
Preventing Test-Unit Contamination
Besides problems with false-negative and false-positive test results, the way integrity test equipment is used during testing also can cause trouble.
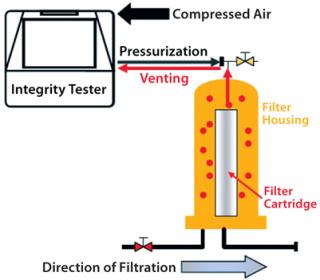
Automatic integrity testers are typically used in biopharmaceutical production for many years. The measuring principle requires pressurization of a filter housing or capsule. As a rule, an integrity test unit pressurizes the filter housing by pumping in compressed air or nitrogen through tubing attached to proportional valves while a pressure sensor monitors the process to ensure that actual pressure corresponds with a programmed nominal pressure.
After a stabilization phase and test itself, the housing is depressurized. In some cases, a vent valve opens in the test unit so test gas flows back through the tubing into the unit, which then releases the pressure. The pneumatic connection between the interior of the integrity test unit and the filter cartridge housing entails a certain risk that under specific conditions, water (or even product or aerosols) will flow back through tubing into the test unit. That can cause moisture and contaminants to build up inside the test unit, and those can then be carried over to the next filter housing during subsequent tests (Figure 2).
Test-unit interiors are not usually sterile, and particles or other contaminants can penetrate from inside them as far as into a filter cartridge housing. And there are applications for which contamination carry-over must be avoided at all costs. There are two possibilities for preventing such cross-contamination: in-line hydrophobic protective filters and external vent valves.
Use of a Hydrophobic Protective Filter in the Test Line: For bidirectional protection, a small, sterile vent filter can be installed directly in the test line between an integrity tester and filter cartridge housing. These are typically 0.2-µm sterilizing-grade membrane filters made of hydrophobic materials such as polytetra-fluoroethylene (PTFE). Test gas is automatically sterile after passing through such a filter, which reliability retains particles as well. The material not only constitutes a sterile barrier, but also provides reliable protection against passage of aqueous liquids, enabling bidirectional protection in the tubing.
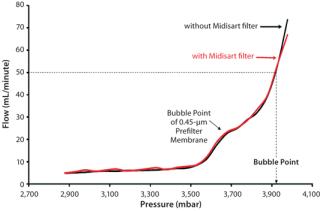
But it must be ensured that such test-line filters will not adversely affect test results. That can be demonstrated even with a small Midisart 2000 vent filter (0.2 µm PTFE, filter area 20 cm2). Integrity tests of sterilizing-grade filters with and without such a protective filter delivered nearly identical test results. Ultimately, the air filter must be sized so that air flow during the integrity test phase is not impeded. For example, a sterilizing-grade Sartopore 2 filter (10 in., 0.2 µm) has a diffusion limit of 18 mL/min at a test pressure of 2,500 mbar. At a differential pressure of 100 mbar, the Midisart filter permits a flow rate of ∼8 L/min. So using such a hydrophobic venting filter in the test line does not limit air flow during integrity tests and thus will not affect results.
Using protective filters is unproblematic even for bubble-point tests. Although the air-flow rate overproportionately increases once a bubble point is attained, that rate remains well below the critical limit. This is demonstrated by testing the same sterilizing-grade filter with and without a protective filter in the test line (Figure 3). The two curves are nearly congruent, so the integrity test unit measures the same bubble point.
So including a filter in the test line does not have any limiting effect. Relatively large filter systems (such as multiple-round housings) may need larger vent filters. But such filters can become blocked if water collects on their membranes. For that reason, during air-filter installation, users must ensure that all condensed water can drain off on its own (by gravity).
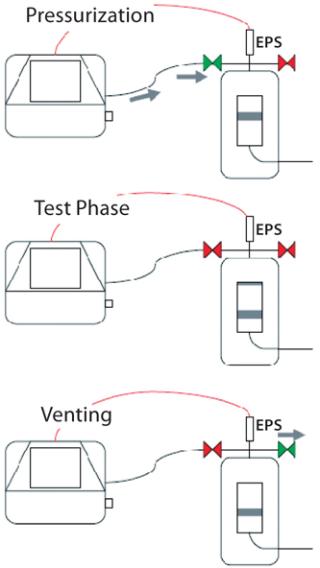
Using External Vent Valves: If problems with liquid back-flow are frequently encountered, blockage of the protective air filter may make it advisable to use external vent valves instead. These are installed on a filter cartridge housing for automatic control by an integrity test unit. During venting (which is necessary once the test phase has ended), the internal vent valve in the test unit will remain closed, and only its external valve will open directly on the housing. So overpressure in the housing is released only through the external valve, and all liquids and aerosols carried along the air stream cannot flow back into the test unit.
A two-valve solution offers even greater security and reliability: One external valve is installed as described above. And a second valve is integrated between the test line and the filter cartridge housing to act as a block valve, opening only during pressurization and remaining closed during both the test and vent phases. This requires use of an external pressure sensor on the filter housing because the internal test-unit sensor can no longer measure any pressure inside the housing due to the closed block valve. In either case — using one external valve or a two-valve installation — the integrity test unit automatically controls the valves so no operator intervention is required (Figure 4).
Using external valves is excellently suited to preventing test gas (and moisture) in the filter housing from flowing back into the integrity test unit. However, such valves do not initially hinder particles that originate from inside the integrity tester from being carried into the filter housing along with the gas stream during pressurization. Ultimately, external valves can be used in combination with an installed hydrophobic protective filter that can retain such particles.
What Can You Do About Contamination?
When integrity test units are operated over years, not even external valves can completely prevent particles or similar contaminants from becoming deposited inside them. Advanced integrity testers feature specific cleaning functions that completely remove such contaminants from the interior surfaces or pneumatic components. But that limits the use of compressed air for reliable removal of particles. Any contaminants that adhere to interior surfaces cannot be reliably eliminated by pumping in compressed air alone.
However, it is possible to clean the pneumatic components inside an integrity tester during a test using liquids that will remove contaminants at their source. Depending on the particular integrity tester model, even aggressive cleaning agents (such as NaOH) can be used and then removed by flushing with pure water. After subsequent drying by compressed air, the integrity tester is again ready to operate immediately for regular testing (Figure 5).
That option for intensive cleaning makes integrity testing considerably more reliable and eliminates the need for expensive servicing. Even without regulatory requirements for
such cleaning procedures, it is advisable to clean integrity testers this way once per year as part of regular maintenance and calibration. Such preventive cleaning substantially improves hygiene and microbiological safety during integrity testing.
Problems with Manual Data Recording
Besides the integrity test run itself, reliable recording of test data is essential. The actual test results must be logged, including lot and individual serial number of each filter element. Errors are frequently made in routine testing mainly when an operator reads and enters multiple-digit lot numbers, especially because imprinted sequences of numbers are more often than not difficult to read. High-contrast laser imprints allow numbers to be read without much problem.
A more reliable method is to have such relevant information automatically transmitted to the integrity tester by a bar-code scanner. Bar codes or data-matrix codes can be transmitted quickly — and, above all, error free. Such information should be inserted logically in the corresponding comment spaces provided by an integrity tester program. Such a system enables scanned information to be logically processed.
Some advanced integrity testers permit hundreds of different test programs to be saved in memory. Even though such high numbers are seldom needed, several dozen different integrity test programs are often used. To test a specific filter cartridge, an operator selects the correct test program from the tester’s database. Here again, operating errors cannot be ruled out. Automatic test program selection increases reliability. If a bar code has already been used to create a special test program, then the integrity tester can automatically select the correct test program when that code is scanned again. Such automation saves time while significantly increasing reliability during daily routines.
Ultimately, a bar-code scanner is merely a different means of entering data. Other than speed, its major benefits coming in error-free reading of numbers and the possibility of combining further intelligent functions.
Practice Prevention
Numerous factors can affect the accuracy of a filter integrity test. So it is important to rule out avoidable errors before testing to prevent unnecessary and time-consuming retesting. Advanced integrity testers provide a number of functions that make filter integrity test procedures more efficient, faster, and more reliable.
Author Details
Jens Meyer is product manager of integrity testing and air filtration at Sartorius Stedim Biotech GmbH, August-Spindler-Straβe 11, 37079 Göttingen, Germany; 49-551-308-0, fax 49-551-308-3289; jens.meyer@sartorius-stedim.com; www.sartorius-stedim.com
REFERENCES