Increasingly efficient bioreactors allow biopharmaceutical manufacturers to achieve higher cell densities. That improved upstream efficiency has led to new purification challenges resulting from high product and contaminant concentrations as well as complex components. Therefore, harvest and clarification techniques are evolving to incorporate feed pretreatment, flocculation, and different filtration technologies such as normal-flow, tangential-flow, and depth filtration. The objective is to increase process capacities and filtrate quality, ultimately reducing biomanufacturing costs.
New strategies for clarification of recombinant proteins (in particular, monoclonal antibodies) and methods of pretreatment can improve clarification efficiency. Such approaches lead to better purity of the obtained filtrate, improving the overall efficiency of downstream purification steps that follow.
Traditional Methods
Using traditional expression systems, bioreactors can support cell densities ranging from 10 to 50 × 106 cells/mL, which allows for product concentrations of ~2–3 g/L. To collect the active intra- or extracellular molecules at the end of such a cell culture, cells may be disrupted (intracellular products and most microbial systems) or discarded (extracellular products and most animal-cell systems). Generated cell debris typically fall below 10 µm in size.
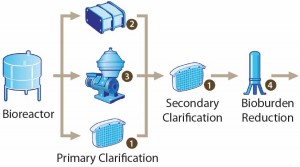
A clarification filtration train clarifies those complex mixtures. Centrifugation or primary filtration is followed by secondary filtration (e.g., using tangential-flow and depth filters) before purification involving chromatography (Figure 1). With a solid load of 3–5% of the volume, such a filtration train results in a typical active product yield >85%.
Tangential-flow filtration (TFF) is used in microfiltration mode (open cut-off from 300 kD to 0.65 µm). It requires thorough process monitoring of pressures, feed flow, flux, and so on. Therefore, TFF is more delicate to use than normal-flow filtration (typically involving depth filters).
When centrifugation is used (often a stacked- disc centrifuge), an intermediate tank is necessary before secondary clarification because centrate flow will be discontinuous. Short-solid discharge phases (cells and their debris) break the centrate flow. Continuous-flow centrifugation can be applied, and downstream steps will have to accommodate its imposed flow rate.
Depth filters are traditionally made of cellulose fibers and an inorganic filter aid (diatomaceous earth) assembled in a lenticular or flat self- contained module (1). Such filters ensure a normal- flow separation of particulates and solutes through two main mechanisms: sieving and adsorption. Based on size exclusion, sieving retains rigid particulates at the surface and depth of a filter. Adsorption works independently from size. It is based on interactions between filtration media and particulates through ionic attraction and hydrophobic or electrostatic forces.For bioreactors with a volume <2,000 L, depth filters represent a significant cost advantage for primary clarification and remain the simplest option.
Today’s Challenges
A decade ago, 200-mg/L antibody concentrations in bioreactors were considered excellent. Today, bioprocessors aim for 10-g/L concentrations, with cell densities >20 Ă— 106 cells/mL. Higher densities necessitate more challenging purification steps (chromatography, TFF), particularly when solids are >10% of the process volume.
Increased biomass naturally leads to higher contaminant loads (2). During primary clarification, with a greater proportion of solids/particulates to eliminate, a filtration cake builds at the surface of traditional depth filters. Their capacity is greatly reduced (<50 L/m²) because their depth is underused for sieving and adsorption (Figure 2). The filter area needed for primary clarification thus becomes unsuitable for industrial use.
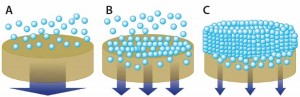
Figure 2: The combination of inefficiently used depth media (a) and formation of additional resistance layer (c) reduces capacity. Optimization of media pore-size distribution using graded depth-filter media and particle-size distribution using pretreatment improves depth-filter performance with higher capacities and lower turbidity (b).
New methods are thus being developed for more efficient primary clarification, combining feed pretreatment with next-generation depth filters.
New Clarification Strategies
To improve the efficiency and capacity of primary clarification by filtration and include single-use technologies, two parameters can be modified and combined: particulate-size distribution in feeds and filtration media structure.
Feed pretreatment is a well-known technique widely applied to wastewater treatment to build a “sludge” and remove biomass. In bioprocessing, pretreatment of bioreactor output causes a shift in average particulate size (>10 µm), which eases particle removal by normal-flow filtration on new depth filter media.
Determining the right pretreatment agent depends on the nature of the protein of interest, contaminants in the feed, and further purification steps downstream. To assess pretreatment efficiency during development, several parameters can be measured: e.g., turbidity in supernatant and particulate-size distribution (for which a Malvern Mastersizer analyzer is useful). Filter capacity is also assessed for both clarifying filters and downstream filtration (e.g., in secondary clarification or bioburden reduction). Product concentration and yield are also indicators for assessing the effects of a pretreatment step on product quality.
Pretreatment options include acids such as acetic acid; salts such as (NH4)2SO4, K2SO4, and KH2PO4; cationic polymers such as chitosan; and other polymers such as pDADMAC and polyethylene glycol (PEG) (3, 4).
Acid treatment is the simplest method. The pH level of a process solution is lowered to 5 (optimized during trials) after addition of an acid, thus modifying the charges of the solutes and leading to aggregation or precipitation of medium-sized particulates (20–30 µm), which are then retained by filtration media. From a regulatory perspective, no agent removal is necessary because only pH is modified, and it is generally readjusted after filtration.
Using salts for protein purification by precipitation is a broadly applied method in biochemistry. The salts interact at the surface of contaminants, decreasing their solubility in the solvent (water) and leading to their precipitation. However, both this method and the pH adjustment described above can denature a protein of interest, thus decreasing its stability and/or yield.
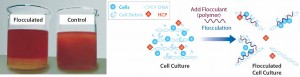
Cationic polymers lead to flocculation. A flocculation agent (the polymer) acts as a “binder” to aggregate contaminants present in a solution, causing formation of a floc (Figure 3). Polymers are nonspecific, binding cell debris, host-cell proteins, nucleic acids, and other contaminants. Flocs are generally larger (30–60 µm) than particulates obtained through acidification. The success of a flocculation step depends mainly on polymer dosage. Removal of that polymer has to be validated because, from a regulatory perspective, free polymer is considered a contaminant.
Depth Filters: Filters for primary clarification of pretreated feeds are constructed to optimize use of their depth (sieving) while keeping a good adsorption capacity. Clarisolve depth filters from EMD Millipore are specifically designed for that purpose (5). The filter media are made of polypropylene with cellulose fibers and/or diatomaceous earth, depending on the grade.
Improved capacity of Clarisolve filters eliminates the need for primary clarification by centrifugation, which reduces capital investment and equipment maintenance while allowing for implementation of single-use technologies. In addition, primary and secondary clarification may be condensed into one step depending on pretreatment efficiency. That can significantly reduce both footprint and depth filtration area — by three to five times — from those of traditional media (Figure 4).
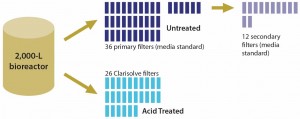
Figure 4: Comparing filtration area required for primary clarification of treated (acid precipitation) and untreated feed
Required flushing volumes before processing also may be significantly reduced, depending on the nature of the media. For example, the layers of polypropylene media that make up Clarisolve 60HX-grade filters reduce flushing volumes by ≤90% (down to <25 L/m² to reach TOC ≤ 3 ppm) compared with media made of cellulose fibers and inorganic filter aids. This can contribute to a more efficient and economic clarification of high–cell- density bioreactors using largely single-use technologies and improving the efficiency of downstream purification steps.
Breaking the Bottleneck
Increasingly efficient bioreactors are enabling higher cell densities. Treatment of their output requires new clarification strategies to increase the efficiency of downstream purification. New clarification methods can reduce filtration area, time, and steps necessary for filter preparation (including flushing). And cleaning requirements can be minimized with single-use technologies. Specific strategies include feed pretreatment and using a macroscopic floc and filtering on media developed for optimum depth use. Compared with a traditional filtration train, these new strategies can improve filtrate purity and increase process capacity to ameliorate the downstream bottleneck.
References
1 Wang A, Lewus R, Rathore AS. Comparison of Different Options for Harvest of a Therapeutic Protein Product from High Cell Density Yeast Fermentation Broth. Biotechnol. Bioeng. 94(1) 2006: 91–104.
2 Zhao X, et al. Developing Recovery Clarification Processes for Mammalian Cell Culture with High Density and High Solid Content. 243rd ACS National Meeting and Exposition, San Diego, CA, 2012.
3 Riske F, et al. The Use of Chitosan As a Flocculant in Mammalian Cell Culture Dramatically Improves Clarification Throughput Without Adversely Impacting Monoclonal Antibody Recovery. J. Biotechnol. 128(4) 2007: 813–823.
4 Kang YK, et al. Development of a Novel and Efficient Cell Culture Flocculation Process Using a Stimulus Responsive Polymer to Streamline Antibody Purification Processes. Biotechnol. Bioeng. 110(11) 2013: 2928–2937.
5 Singh N, et al. Clarification of Recombinant Proteins from High Cell Density Mammalian Cell Culture Systems Using New Improved Depth Filters. Biotechnol. Bioeng. 110(7) 2013: 1964–1972.
Sarah Le Merdy is a field marketing specialist in core field marketing for purification and BMT Process Solutions at Merck Millipore, sarah.lemerdy@merckgroup.com. Clarisolve is a registered trademark.