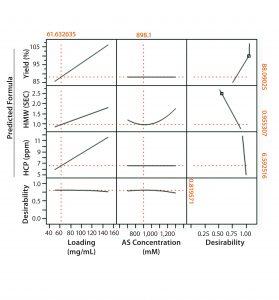
Figure 1: Full-factorial DoE (molecule 1) with three levels of ammonium sulfate concentration (0.7M, 1M, and 1.4M) and membrane loading (50 mg/mL, 100 mg/mL, and 150 mg/mL). Responses studied were percent yield in flow-through product, clearance of high–molecular weight (HMW) impurities, and HCP clearance.
Given paradigm shifts in the biopharmaceutical industry over the past decade, product development timelines are squeezed as the number of molecules entering clinical development continues to increase. Manufacturing facilities, especially those supplying clinical trial materials, have had to adapt to this trend. One popular approach is to have fully disposable equipment that allows for quick product changeover and flexible manufacturing capacity to respond to variable clinical demand. Although many facilities-related technologies exist to support that concept (e.g., disposable probes and mixers, single-use skids, and bioreactor bags that support extended production cultures), process design and development also should evolve to embrace the flexible manufacturing concept.
In that regard, single- or limited-use stationary phases in the form of membrane chromatography (MC) units offer attractive alternatives to reusable chromatography columns. Several ion-exchange MC units have been available commercially for some time: e.g., Mustang Q systems (Pall), NatriFlo HD-Q membrane chromatography (Natrix Separations), and Sartobind STIC and Sartobind Q units (Sartorius Stedim). The latter provide strong anion-exchange functionality and have been used in many processes (including at large scale) as a final polishing step for removing residual DNA, host cell
protein (HCP) impurities, and adventitious viruses (1, 2).
Membrane chromatography is particularly well suited for clinical manufacturing facilities. Unlike commercial facilities that make products using a validated process, quick turnaround time is key to flexibility for clinical manufacturing facilities. MC units are the preferred choice to enable that. Such facilities typically operate in “campaign” mode, with a certain number of lots made over a fixed period before a facility is turned over to manufacture another clinical candidate. In these scenarios, using multiuse chromatography resins is not cost effective because concerns over cross-contamination and product carryover preclude their application to multiple products. Validation costs are minimal or nonexistent for media that can be used at most for a handful of times for a single product. Given that MC units can operate at very high flow rates (unlike column chromatography), throughput and process cadence are increased, further aiding in rapid product changeovers for a manufacturing facility. Finally, computer simulations of a fully disposable 2,000-L facility have shown that water consumption is lowered compared with column chromatography (3). The major disadvantage of membrane adsorbers is that they suffer from a relatively low binding capacity; however, significant progress has been made over the years especially in engineering design to increase membrane capacities.
We considered one such unit (Sartobind phenyl) that is engineered specifically to have a relatively higher binding capacity (4, 5). If process design involves flow-through chromatography mode — with impurities binding and product flowing through — the capacity of these membrane adsorbers should be sufficient to clear impurities. Because the capacity of membrane adsorbers is small, these units often are used in a polishing step to clear residual impurities. Here we demonstrate our successful use of a phenyl membrane unit for impurity removal of two antibody-based products.
Below we present an example two-step purification process in the manufacture of a full-length monoclonal antibody (MAb) and an Fc fusion protein — the representing a challenging case with a starting high–molecular-weight (HMW) content of about 9%. Although not explicitly considered herein, this process could be adapted to include an additional intermediate processing step with another charge-based MC step to provide an orthogonal approach for additional robustness. This process can be implemented easily in a disposable facility to provide flexibility in manufacturing. If used as a platform process, affinity capture followed by hydrophobic-interaction membrane chromatography (HIC MC) can fit well into a facility based on single-use technology.
Materials and Methods
All load materials were purified using a preparative affinity chromatography capture step (based on MabSelect SuRe or MabSelect SuRe LX resins from GE Healthcare). We obtained phenyl Sepharose FastFlow resin from GE Healthcare. Before loading process fluid on the MC unit, we adjusted the load to an appropriate salt concentration using stock solutions prepared with respective salts. The phenyl MC unit we used came from Sartorius (Phenyl Nano 3 mL), and we connected it to a GE ÄKTA Avant 150 system using supplied fittings. Buffers and stock solutions prepared in-house used chemicals obtained from Sigma and were 0.22-µm filtered before use.

Table 1: Step performance summary for molecule 1 with the step running at the optimum conditions (0.9M ammonium sulfate concentration and 60 mg/mL membrane loading) — an average of duplicate runs
We adjusted the protein A eluate’s pH level upward using either Tris or HEPES buffer. To adjust the final salt concentrations of our load materials, we used stock solutions of ammonium sulfate in each buffer. At the end of each run, we cleaned and sanitized our MC unit according to the manufacturer’s recommendations before reuse. We based all residence times and flow rates on the manufacturer’ recommendations, as well. To measure protein concentration, we used a DropSense benchtop spectrophotometer from Trinnean. For size-exclusion chromatography (SEC) analysis, we used either Waters bridged ethylene hybrid (BEH) or Tosoh Bioscience columns on a Waters instrument. And to measure residual HCPs, we applied Cygnus Technologies enzyme-linked immunosorptive assays (ELISAs) based on the manufacturer’s instructions.
Table 2: Step performance summary for molecule 2 with the step running at optimum conditions (0.575 M ammonium sulfate concentration and 40 mg/mL membrane loading) — an average of duplicate runs; the resin loading specification is 55 g/L.
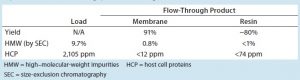
Table 2: Step performance summary for molecule 2 with the step running at optimum conditions (0.575 M ammonium sulfate concentration and 40 mg/mL membrane loading) — an average of duplicate runs; the resin loading specification is 55 g/L.
Results and Discussion
For each molecule of interest, we chose HIC MC operating conditions using statistically designed experiments. Actual process conditions for initial screening were based on molecule 1’s (the MAb’s) molecular properties and molecule 2’s (the Fc fusion protein) existing HIC column step. With design of experiments (DoE), we determined a preliminary operating range for the two most important process variables: membrane loading and ammonium sulfate concentration of the load. The ranges we studied were 0.7–1.4M ammonium sulfate and 50–150 mg/mL membrane volume (molecule 1) and 0.45–0.75M ammonium sulfate and 40–100 mg/mL membrane volume (molecule 2).
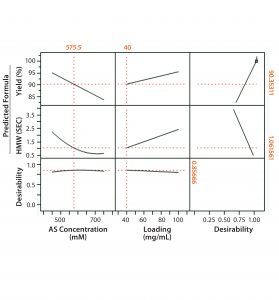
Figure 2: Full-factorial DoE (molecule 2) with three levels of ammonium sulfate concentration (0.45M, 0.6M, and 0.75M) and membrane loading (40 mg/mL, 70 mg/mL, and 100 mg/mL). Responses studied were percent yield in flow-through product, HMW clearance, and HCP clearance.
Figures 1 and 2 show DoE results for molecules 1 and 2, respectively. Based on a prediction profiler, we performed experiments in duplicate at the optimal conditions recommended by our statistical model. Tables 1 and 2 summarize the yield and clearance of impurities (HMW impurities and HCP). The yield for both molecules from this step is in the 80–90% range. And as can be expected with HIC media, the HMW impurities bind more strongly to the column and are enriched in the strip fraction (Table 1).
For flow-through modes of operation, increased loading generally results in higher yields, assuming that MC capacity for impurity clearance is not exceeded. However, the DoE results indicate a tradeoff between yield and HMW clearance, one of which can be increased at the expense of the other depending on requirements. We also investigated the effect of membrane loading on HMW clearance for molecule 1. As can be expected, with an increase in load from 50 to 150 g/L the HMW clearance decreased from 6Ă— to 2Ă—. Membrane loading did not affect HCP clearance (Figure 3).
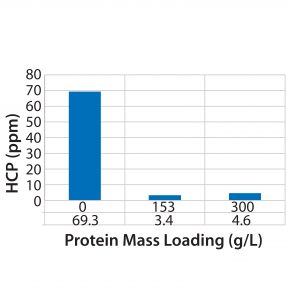
Figure 3: Effect of increased loading on a phenyl chromatography membrane unit regarding HCP clearance (molecule 1)
In the case of molecule 2, the HIC MC conditions are comparable to those of an existing HIC step operated in similar flow-through mode using column chromatography — the main difference being higher loading on the column operation because of the inherently higher capacity of resins. Because the starting impurity levels for the Fc fusion protein are high, our options are limited for increasing the load while maximizing yield and impurity clearance in this case (again because of HIC MC’s limited capacity).
Finally, we explored the idea of reusing the membrane module multiple times. After performing a maximum of five cycles, we found no deleterious effects. Even though we studied only five total uses, we believe that the membrane may be able to be reused more times. Chromatograms matched exactly from all five cycles (Figure 4). From those chromatograms, it is evident that duration/run times are very short, indicating a rapid cadence for this unit operation. The example below shows how these results can be applied to a hypothetical case.
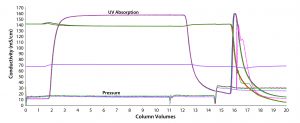
Figure 4: No significant chromatogram shifts are seen with membrane reuse for a total of five cycles (overlay of chromatograms after five runs).
Application
For MAbs and other molecules containing Fc structures, a standard platform process for purification consists of an affinity capture step followed by an ion-exchange chromatography step (6) — and optionally, a final charged membrane chromatography step. Because protein A can remove only HCP impurities, a dedicated chromatography step is needed to remove HMW impurities in the above scenario. The final IEX MC step has very limited capacity for HMW removal. Charged MC can provide clearance of charged impurities such as DNA, some HCPs, and viruses, but it does not have much capacity for HMW clearance because they are hydrophobic. Our results have shown that HIC MC can be used as a polishing step to remove aggregates (usually thought of as hydrophobic). That opens up the possibility of using HIC MC to replace a HMW-removing column operation. If needed, another IEX membrane polishing step could be added to provide additional impurity/virus clearance.
Given the availability of HIC MC units in different sizes and formats, this two-step (or three-step if an additional IEX MC is used for secondary polishing) process can be implemented easily in a 2,000-L facility based on single-use technology. For a 2,000-L bioreactor, assuming a titer of 1 g/L and full recovery (100%) during both the harvest and protein A steps, about 2 kg of material would need to be processed across the HIC MC unit. With a 50 mg/mL loading, that would require 40 L of HIC MC media. Phenyl Sartobind membranes come in 5-L “jumbo” units or cassettes (which can require a holder and skid, incurring additional capital cost). Because each HIC MC unit can be reused, two 5-L units could be operated in parallel (providing a total 10-L capacity), which would require four cycles to process the entire 2,000-L bioreactor volume.

Figure 5: Possibility of using hydrophobic interaction membrane chromatography as a final polishing step after affinity capture (left) or as a second polishing step following initial polishing with ion-exchange MC (right)
If the worst case (molecule 2) is excluded, even at 150-mg/mL loadings we observed HMW levels of ~2% and HCP levels of 17 ppm in the HIC MC flow-through product. That may be adequate purity for early phase clinical material. In this case, just one 5-L cartridge can be used and cycled three times to process a 2,000-L bioreactor’s output. Finally, although not specifically described as such herein, HIC MC also can be used as the second polishing step (Figure 5) in a currently well-established paradigm of affinity capture followed by ion-exchange MC to add orthogonality and robustness to impurity clearance.
Polishing Potential
We studied two cases (including a “worst-case” scenario with high HMW impurity levels) to evaluate phenyl Sartobind membrane chromatography as a polishing step in a platform purification process. The phenyl MC unit removes HMW aggregates about sixfold in the case of full-length MAb and eight- to ninefold for a fusion protein. HCP removal also is significant, bringing down HCP levels to <100 ppm and <10 ppm in the case studies, respectively. Both impurities represent the major focus for a process development scientist involved in designing a purification scheme. Clearance levels obtained here should be acceptable for early clinical phase trials. Yield in both cases exceeded 80%.
This work shows the potential of HIC membrane chromatography as a final polishing step in a two-step purification process, giving both HMW aggregates and HCP clearance. Alternatively, it can be applied as an orthogonal polishing step to improve robustness of a two-step purification process using affinity capture followed by a charge-based MC step. Ion-exchange MC has been firmly established as a final polishing step in many processes. It is demonstrated as an excellent step for removal of HCPs and DNA. Our work has shown that phenyl Sepharose MC can provide another option to ion-exchange MC for impurity removal.
References
1 Mora A, et al. Disposable Membrane Chromatography. BioProcess Int. 4(4) 2006: 38–43.
2 Zhou JX, Tressel T. Basic Concepts in Q Membrane Chromatography for Large-Scale Antibody Production. Biotechnol. Progr. 22(2)2006: 341–349; doi:10.1021/bp050425v.
3 Lim J, et al. Technical and Economic Benefits of Membrane Chromatography During Polishing Steps. BioPharm Int. August 2010.
4 Fraud N, et al. Hydrophobic-Interaction Membrane Chromatography for Large-Scale Purification of Biopharmaceuticals. BioProcess Int. 7(6) 2009: 30–35.
5 Ebert S, Fischer-Frühholz S. Efficient Aggregate Removal from Impure Pharmaceutical Active Antibodies. BioProcess Int. 9(2) 2011: 36–42.
6 Shukla AA, et al. Downstream Processing of Monoclonal Antibodies: Application of Platform Approaches. J. Chromat. B 848(1) 2007: 28–39; doi:10.1016/j.jchromb.2006.09.026.
Formerly a scientist II and downstream lead at BMS, Mukesh Mayani is a development engineer in cell and gene therapy development at GE Healthcare. Corresponding author Chittoor Narahari (process.scientist@gmail.com) is principal engineer at Moderna Therapeutics in Cambridge MA (formerly a senior scientist in biologics process development at BMS). Vivekh Ehamparanathan is associate scientist II in biologics process development at Bristol-Myers Squibb, 38 Jackson Road, Devens MA 01434. Hemanth Kaligotla is a product marketing manager, and Susan Martin is a purification application specialist at Sartorius Stedim North America.
