This educational podcast, “The Evolution of Culture Systems for Viral Vector Production: Advantages, Challenges and Cost Considerations,” recently published by Cell and Gene Therapy Insights, discusses in detail the pros and cons of viral vector production in adherent and suspension cell culture. This special report illustrates how Pall Biotech’s iCELLis 500+ bioreactors and Allegro STR bioreactors can bolster adherent and suspension culture, respectively, for viral vector production. Fill out the form below to read the complete report and learn more now.…
Upstream Single-Use Technologies
Implementation of Single-Use Miniature Bioreactors to Support Intensified Cell Culture: Using Functional Performance Indicators to Assess a Small‑Scale Model
Changes to bioprocessing in the biopharmaceutical industry are driven by the need for increased speed, lower cost of goods (CoG), and greater flexibility (1). To meet these challenges, the industry is adopting strategies that include intensified processing. During the initial stages of intensified processing, it is essential to identify the most productive and/or stable clones for use before starting pilot-scale studies. That requires screening large numbers of clones and then further testing the most promising ones in benchtop bioreactors. The…
Innovative Strategies for Cell Culture Media Preparation
Although the handling and preparation of cell culture media can seem routine, a number of risks are associated with such operations. Identification and mitigation of associated risks can help ensure consistency of performance, minimize likelihood of contamination, and protect employees while enabling greater efficiencies in upstream processes. Here we describe a number of strategies for reducing risks and streamlining media-related workflows. Simplifying Handling of Cell Culture Powders Media preparation typically is quite labor intensive and poses risks related to containment…
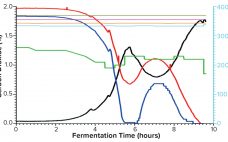
Comparative Study of Single-Use and Reusable Fermentors: Production of Recombinant Proteins Through Bacterial Fermentation
Single-use bioreactors have become widely accepted and well established for cell culture applications in the biopharmaceutical industry for over a decade (1). Abbott Diagnostics has moved into this technology already for commercial production of some biologic molecules. However, single-use systems (SUSs) are rarely available for microbial applications, mostly because of the technical challenge in designing cost-effective SUSs that can meet high oxygen transfer needs and remove excessive heat generated during fermentation. Thus, an important part of our biologics manufacturing —…
Production of Transient Lentiviral Vectors in HEK 293T Cells: Cultivation on Fibra-Cel Disks in a Single-Use, Packed-Bed, Stirred-Tank Bioreactor
Although demand for lentiviral vectors (LVs) for cell and gene therapy is increasing, the standard two-dimensional culture systems used to produce LVs present significant disadvantages. Current bottlenecks in LV production are caused mainly by such disadvantages. Switching to use of bioreactors can eliminate those problems because bioreactors offer the benefits of process automation, tight regulation of production conditions, and reduced labor input. The study reported herein was carried out by the group of David Parsons at the University of Adelaide.…
A Shift of Mindset: How Single-Use Systems Influence Bioprocess Engineering and Project Execution
In past decades, the focus in bioprocess engineering was on traditional stainless steel project management, which is highly dependent on established project schedules. Many milestones, tasks, and deliverables during basic and detailed design and execution were implemented in the same tried and tested way by engineering, suppliers, and biopharmaceutical companies. Making decisions was complicated by alignment with long lead items. With the introduction of single-use (SU) technologies, the transition in execution and optimization of project management only just has been…
Single-Use Technology in Upstream Processing: A Roundtable Discussion
The Sartorius upstream portfolio addresses key strategic challenges facing the biopharmaceutical industry: Increased speed to clinic/market and lowered capital costs, with improved process control. Fully scalable, proven process solutions for cell line, media, and process development through commercial manufacturing accelerate upstream development and simplify manufacturing. Novel high-throughput development tools for intensified processes incorporate the latest in process analytics, multivariate data analysis (MVDA), and design of experiments (DoE) software tools. These tools are designed to compress development timelines and to scale…
Scientific and Technological Advancements in Applications of Single-Use Technology: A Conference Report
Single-use technology (SUT) has been used increasingly both in clinical and commercial biomanufacturing (1). Proven major advantages include relatively low capital investment, elimination of batch-to-batch cross contamination and reuse cleaning validation efforts, flexibility in manufacturing, and shortened product lifecycles. However, some challenges and barriers to implementation remain: Consumables costs are increasing. Specific regulatory guidance is lacking, as is component interchangeability and standardization. And few if any leak-proof components/systems are available. International groups and associations focused on setting best practices and…
A Brief History of Adherent Cell Culture: Where We Come From and Where We Should Go
In the past 20 years, novel therapeutics have become a major segment of biopharmaceutical research and development, particularly for immune disorders and cancer. Progress in gene therapies could bring cures for once deadly and debilitating genetic disorders such as hemophilia or muscular dystrophy. Biologic drug products offer potential treatments that have not been possible with traditional (chemistry-based) approaches. But such products also are more difficult to produce cost effectively at an industrial scale because of the intricacies associated with biological…
Cell Culture Scale-Up in Stirred-Tank Single-Use Bioreactors
Bioprocess development usually is carried out in systems with small working volumes. This helps save time and resources because, at small scale, several experiments can be conducted in parallel. Costs for media are kept low, and relatively little laboratory space is required to operate small-scale bioreactors. But over the course of development, biopharmaceutical companies need more material for characterization, trial runs, and finally for commercialization. They transition to bench scale and then up to pilot or production scale with the…