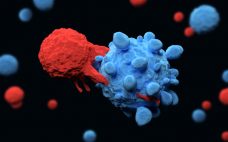
There are now 60 different centers certified to administer CAR-T therapy Yescarta says Gilead Sciences says, which plans to add more as further indications are approved. In October 2017, the US Food and Drug Administration (FDA) approved the second chimeric antigen receptor T-cell therapy in the form of Yescarta (axicabtagene ciloleucel), developed by Kite which had recently been acquire by Gilead Sciences for $11.9 billion (€10.2 billion). Yescarta is made by isolating peripheral blood mononuclear cells – including T-cells –…

Therapeutic Class
Sanofi and Regeneron gain approval for skin cancer MAb
The US FDA has approved skin cancer drug Libtayo, a programmed cell death protein-1 (PD-1) inhibitor developed through a Sanofi and Regeneron collaboration. In 2007, Sanofi teamed up with Regeneron Pharmaceuticals to discover, develop, and commercialize fully-human therapeutic antibodies. While the discovery collaboration ended last year, Sanofi still owns a 22% stake in Regeneron. The the firms also continue to develop and commercialize a number of monoclonal antibodies, including its metastatic cutaneous squamous cell carcinoma (CSCC) product cemiplimab which the two…
Double deal with Asterias gives Novo Nordisk CA stem cell plant
Novo Nordisk will pay Asterias $2 million to lease a facility in Fremont, California for the supply of stem cell-based therapies. An manufacturing tech licensing deal is also an option. Danish drugmaker Novo Nordisk intends to set up manufacturing capabilities for its clinical phase stem cell therapies for type 1 diabetes and other chronic diseases from 2019 onwards after subleasing the plant in California from Asterias Biotherapeutics. The 44,000 square feet current good manufacturing practices (cGMP) facility is used as…
Pandion selects Just to cut cost of bispecific Ab development
Just Biotherapeutics says smart design and optimization of the molecule and the manufacturing process will speed the development of Pandion Therapeutics’s lead bispecific antibody PT101. Just Biotherapeutics has entered into a master services agreement for the development and clinical manufacturing of Pandion Therapeutics’ lead candidate. Pandion is a biotech located in Cambridge, Massachusetts developing bispecific antibodies for the tissue-specific treatment of patients with autoimmune and inflammatory diseases and organ transplants. The firm completed a $58 million (€50 million) Series A…
Alexion refilling pipeline through potential $1.2bn Syntimmune buy
The acquisition of early-phase biotech Syntimmune is “part and parcel” to Alexion Pharmaceuticals’ plan to build up its pipeline, says the firm’s CFO. Alexion Pharmaceuticals has entered into an agreement to buy Syntimmune for an upfront payment of $400 million (€342 million), though milestone payments could see the firm shell out a further $800 million. The deal brings Alexion SYNT001, a humanized monoclonal antibody candidate that inhibits the interaction of FcRn with Immunoglobulin G (IgG) and IgG immune complexes. The…
Gene editing deal leaves Thermo Fisher ‘unique in offering TALENs and CRISPR’
Thermo Fisher Scientific has increased its gene editing technology offering by licensing CRISPR/Cas9 technologies from the Broad Institute and ERS Genomics. Thermo Fisher will now be able to offer its biopharma customers several genome editing offerings through the in-license deals. “Genome editing has unlocked the door for researchers to begin understanding gene function, which is fundamental in unraveling and outlining basic biological processes and disease pathways,” said Helge Bastian, vice president and general manager of Synthetic Biology at Thermo Fisher…
Eli Lilly on M&A: Balancing the science with the shareholders
Many early-phase acquisitions do not make sense for shareholders despite having promising assets, says Lilly’s CFO Joshua Smiley. About a third of Lilly’s pipeline comes from M&A. Speaking to attendees at the Morgan Stanley Healthcare Conference Broker Conference Call this month, Eli Lilly CFO Joshua Smiley said his firm is “interested in doing early stage clinical deals where we see compelling scientific rationale in our key therapeutic areas and that we can upgrade our pipeline.” But deals also need to…
Video news: Pall strategic deals aim to enable biosimilar and novel scale-up
Pall Corporation has inked separate partnerships with cell line and expression tech firms Celltheon and Aetos Biologics. The deals help enable both novel and biosimilar manufacturing, the firm says. At the 2018 BPI Conference in Boston, Massachusetts earlier this month, Pall Corporation announced it had entered into a strategic partnership with cell line and expression technology platform development company Celltheon Corporation. The deal combines Pall’s bioprocessing equipment and consumables with Celltheon’s SMART Expression Platform to offer customers integrated systems…
B-I working with CMO on viral therapies after $245m ViraTherapeutics buy
Boehringer Ingelheim has acquired ViraTherapeutics for €210 million ($245 million) and tells us how it will integrate the viral therapy firm into its oncology business. In September 2016, German biomanufacturer Boehringer Ingelheim partnered with Austrian based ViraTherapeutics GmbH to develop an oncolytic virus therapy platform and bring various programs through the clinic. As part of the deal, Boehringer Ingelheim had the right to acquire its collaborator after the conclusion of Phase I clinical development, something the firm has now undertaken…
Exclusive: Samsung BioLogics poised to enter cell therapy space
After building three large-scale biomanufacturing plants, CDMO Samsung BioLogics has told BioProcess Insider it is adding single-use capacity and is considering expanding into stem cell therapy services. It has been nine years since Samsung BioLogics entered the third-party biologics space, in which time it has brought two commercial biomanufacturing facilities online with a third expected to be fully validated by the end of this year. When operational, the firm’s site in Incheon, South Korea will boast a total of 362,000…