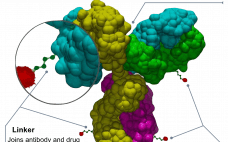
The complex and multiple parts of antibody-drug conjugate (ADC) manufacture means it is integral to choose a CDMO with mAb, small molecule, and bioconjugation expertise, say WuXi Biologics and WuXi STA. The onslaught of ADCs is a hardy perennial in the pages of this publication and others when it comes to predicting the future of medicine. In 2011 when Adcetris (brentuximab vedotin) â then only the second ADC â was approved, it was predicted to be the watershed moment and…

Therapeutic Class
J&J trial pause does not bode well for viral-vector COVID vaccines, analyst
Johnson & Johnson says the pausing of dosing in trials of its COVID-19 candidate is not unusual, but one analyst believes this could have significant negative impact for viral-vector based vaccines. Johnson & Johnson announced at the beginning of the week it has temporarily paused further dosing in all its COVID-19 vaccine candidate clinical trials due to an unexplained illness in a study participant. This included the Phase III ENSEMBLE trial of JNJ-78436735, also known as Ad26.COV2-S, recombinant, which is…
SQZ Biotech lines up $75m IPO for cell therapy R&D
SQZ Biotechnologies says its technology can produce cell therapies in less than 24 hours compared to a month or more for current products and is looking to use IPO proceeds toward the completion of Phase I development of its lead therapeutic candidate. In paperwork filed with securities regulators late last week, SQZ set a preliminary $75 million goal for its IPO. The Watertown, MA-based biotech has applied for a listing on the New York Stock Exchange under the stock symbol âSQZ.â SQZ…
Mustang licenses transduction enhancer for XSCID program
Mustang Bio will use a newly licensed cell transduction enhancer to make MB-207, its candidate therapy for âbubble boy disease.â The US biotech licensed rights to Sirion Biotechâs Lentiboost technology last week, explaining it will apply the transduction enhancer its X-linked severe combined immunodeficiency development (XSCID) program. CEO Manuel Litchman said, âTransduction enhancers were added to the cell processing of the lentiviral gene therapy in 2019 and several advantages have been observed. He added the firm plans to file an…
Navigo and Repligen team on COVID-19 vaccine affinity resin
Protein A-derived affinity ligands against the COVID-19 spike protein coupled to chromatography beads can resolve purification challenges in the development of coronavirus vaccines, say Navigo and Repligen. Bringing a vaccine against COVID-19 to market involves numerous challenges, not least on the manufacturing side. In downstream processing (DSP), the speed of purification and the yield of the purified product are key to ensuring supply of a quality vaccine. As such, bioprocess technology firm Repligen teamed with protein engineering firm Navigo Proteins…
Aji Bio receives FDA approval for commercial oligo process
Ajinomoto Bio-Pharma Servicesâ AJIPHASE Technology has received US FDA approval for an undisclosed commercial oligonucleotide, most likely NS Pharmaâs Viltepso (viltolarsen). The announcement highlights Ajiâs AJIPHASE synthesis technology in its potential to scale up oligonucleotide production. âAJIPHASE is used in the development and cGMP manufacture of large-scale oligonucleotides and peptides,â a spokesperson from the company told this publication. âThe AJIPHASE technology employs the use of an anchor, instead of a resin, which provides a homogeneous mixture. After the reaction occurs,…
Competition for CMOs drove inhouse investment, says Regenxbio
Regenxbio says increasing competition for capacity in the CMO space prompted the adoption of a hybrid gene therapy manufacturing strategy. Industry demand for contract manufacturing organizations (CMOs) with the technical skills required to manufacture gene therapies has increased in recent years. According to the Alliance for Regenerative Medicine, as of Q3 last year, 370 trials involving gene therapies were ongoing, which is an increase on the 351 underway in the equivalent period in 2018. Katie Masterson, director of manufacturing operations…
Orgenesis acquires cell therapy specialist Koligo for $15m
Orgenesis agrees merger with regenerative medicine company, gaining access to a commercial cell therapy, a COVID-19 candidate, and bioprinting technology. The two companies have entered into a definitive merger agreement, which is expected to close by the yearâs end. Orgenesis will pay $15 million (âŹ12.7 million) in shares of its common stock to Koligo Therapeuticâs investors to secure the deal. Koligo possesses one commercially-available treatment, in the form of Kyslecel, which is an autologous pancreatic islet cell therapy used to…
Arranta says GMP plant on track despite pandemic
Microbiome contractor Arranta Bio says construction of its commercial scale manufacturing plant is on schedule despite the coronavirus pandemic. The contract development and manufacturing organization (CDMO) announced the mechanical completion of GMP suites at the facility in Watertown, Massachusetts this week. CEO Mark Bamforth thanked, âOur construction management partner, DC Beane and Associates Construction CompanyâŚand the numerous sub-contractors who have supported Arranta and worked through the pandemic in a safe and efficient manner to keep our commercial-ready manufacturing facility build-out…
Pfizer to make Vivetâs Wilson disease gene therapy for Phase I/II trial
Pfizer will make a candidate gene therapy for the liver condition Wilson disease under an agreement with Vivet Therapeutics. The US pharmaceutical firm will make supplies of the candidate â known as VTX-801 â for a Phase I/II clinical trial due to start early next year. Wilson disease is characterized by the accumulation of copper in tissue. It is an inherited disorder caused by mutations in the gene encoding the ATP7B copper transporter. VTX-801 is an AAV liver tropic capsid…