The partnership looks to develop vaccines using Intravaccâs outer membrane vesicles (OMV) tech and Cristal Therapeuticâs copper-free click chemistry reagent with an initial target being COVID-19. The two companies will work together to develop novel vaccine programs with initial work during the evaluation period taking place at Cristalâs facility, followed by the further work conducted at Intravaccâs facility, both in the Netherlands. Financial details of the collaboration have not been divulged. Initially, the partnership is targeting a vaccine against COVID-19.…

Therapeutic Class
Sanofi: âVaccine heritage puts us in good stead to play in gene therapy spaceâ
Sanofi says it is looking to resolve the âpatchyâ accomplishments in the gene therapy space through a new specialized unit that will leverage technologies from its vaccine division. Sanofi has dabbled in the cell and gene therapy over the past decade but is yet to make its mark. Speaking at the JP Morgan Healthcare Conference this week, CEO Paul Hudson highlighted the importance of the sector going forward but admitted industryâs success in the sector has âbeen patchy.â He told…
Fujifilm invests in third viral vector plant citing âsurgingâ demand
Fujifilm says surge in demand for viral vectors prompted decision to invest in third development and production plant. Fujifilm announced plans to invest $40 million to set up the new plant in in Watertown, Massachusetts last week. The Japanese conglomerateâs contract services unit â Fujifilm Diosynth Biotechnologies â will run the facility when it comes online. In a press statement the CDMOâs CEO Martin Meeson said, âWe are strategically establishing this facility in the greater-Boston area where there is a…
Singapore sets sights on soaring stem cell demand
Singapore’s A*star wants to use micro-carrier tech to capture a share of the global stem cell manufacturing market. Demand for stem cells has increased markedly in recent years, creating a significant opportunity for those willing to innovate to help industry address its manufacturing challenges according to Steve Oh from A*star. âIn a bid to do that, the Singapore government invested $80 million in three programmes to manufacture living cells as medicines,â Oh told delegates at the Cell and Gene Therapy…
French biotech sets up exosome plant to deliver proteins and vaccines
Ciloa says its exosomes can âdeliverâ large molecules and viral proteins to specific organs and is setting up a clinical plant in Montpellier, France. The âŹ1 million ($1.2 million) investment will be used to create an exosome production unit at Ciloaâs site in Montpellier. The facility will be a technological demonstrator that will be the prototype for larger projects of production of customized exosomes, capable of producing around 1,000 doses to support early clinical trials, the firm told us. Exosomes…
Quality cell banks de-risk regenerative medicine development, says consultant
Minimizing variability in cells used to make regenerative medicines is vital but challenging according to an expert, who says a quality cell bank is key. Cell and gene therapies have the potential to revolutionize medicine and allow physicians cure previously untreatable diseases. But developing a process that produces a stable, consistent product is a technical challenge. A major challenge is that the starting materials â the cells from which the medicines are produced â need to be carefully handled says…
rBIO launches aiming to increase manufacturing output of insulin
rBIO plans to lower cost of insulin by 30% through the âhyper-expressionâ of newly-developed strains of bacteria. The start-up company is launched out of San Francisco and arrives alongside the news that it has successfully synthetically produced human insulin, using E. coli bacteria as the host for recombinant DNA synthesis. According to rBIO, the next stages for the biotech are to upscale production of insulin and to identify other prescription drugs that can be manufactured using its approach. Already the…
Eli Lilly $880m Prevail buy a âgood entry pointâ into gene therapy
As Eli Lilly agrees to buy Prevail Therapeutics adding a pipeline of neuroscience assets, CEO Dave Ricks hints at further acquisitions in the gene therapy space. The deal announced yesterday will see Eli Lilly pay approximately $880 million upfront for New York-based Prevail Therapeutics, with a further $160 million set to be paid upon the first approval of a product. Prevail, launched in 2017, is developing gene therapies to slow or stop the underlying disease process for patients with neurodegenerative…
Production begins at vector suite Catalent set up for Passage Bio
Catalent has started making viral vectors to support trials of PBGM01, a gene therapy for the rare lysosomal storage disease GM1 being developed by Passage Bio. The US contract development and manufacturing organization (CDMO) set up a dedicated manufacturing suite at for Passage at its facility in Harmans, Maryland in August. Work since then has focused on bringing the suite online and validating it for the production of the AAVhu68 vector. The vector is used to deliver a modified DNA…
Boehringer Ingelheim re-enters ADC space with $1.4bn NBE buy
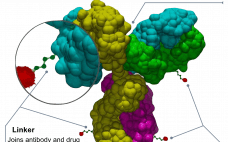
Having already dabbled in the antibody-drug conjugate (ADC), Boehringer Ingelheim has gone full in, acquiring NBE-Therapeutics for âŹ1.18 billion ($1.4 billion). The deal sees German biopharma firm Boehringer Ingelheim agree to buy private Swiss biotech NBE-Therapeutics, adding an ADC technology platform and a lead compound NBE-002 in Phase I clinical studies for triple negative breast cancer and other solid tumors. When the deal closes â expected in Q1 2021 â âNBE Therapeutics with its team of highly qualified scientists will…