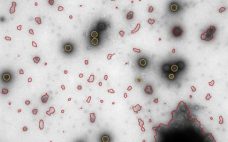
In an October webcast, Vironova’s Josefina Nilsson (head of electron microscopy services) and Gustaf Kylberg (MiniTEM product manager) discussed subvisible particle characterization using the MiniTEM system, which provides both morphology and quantitative data. Nilsson and Kylberg’s Presentation Vironova combines expertise in electron microscopy and image analysis with in-house designed software. Electron microscopy is a good technique for getting to know your product in detail (morphology and other characteristics). After discussions with partners and clients, Vironova worked with Delong Instruments to…
Sponsored Content
Enabling Custom Solutions for Downstream Processing for Future Therapies: AAV Case Study
This webcast features: Orjana Terova, Purification Product Manager, Thermo Fisher Scientific There is a need in the industry for a standard purification strategy for the manufacture of novel molecules including viral vectors and oligomeric therapies. Working with customers, Thermo Fisher Scientific has developed innovative purification resins through the POROS™ custom resin program and CaptureSelect™ ligand technology. This combination of ligand and resin development has allowed for development of platform purification processes that increase productivity and improve product yield for gene therapies.…
The Standardization of Single-use Components for Bioprocessing
As single-use systems become more widely adopted, the focus in the bioprocessing industry is shifting from acceptance of the technology to standardization. While the standardization discussion encompasses many topics (including how products are tested, assembled, etc.), components are a critical area for improvement. With so many ways to apply single-use and hybrid bioprocessing systems, organizations must take action to standardize equipment in order to streamline operations and help reach the full potential of the technology. This paper discusses standardization in…
The Total Cost of Buffer Preparation and Handling
This webcast features: Eric Langer, Managing Partner, BioPlan Associates, Inc. Most bioprocessing facilities consider in-house preparation of buffers and bulk liquids to be a core bioprocessing task. However, some companies are now outsourcing liquid manufacturing, by purchasing ready-to-use materials from vendors or hiring CMOs to prepare these. Buffers and bioprocessing liquids are one area of upstream production operations that is seeing an increase in outsourced operations. Bulk liquids can be a bottleneck in downstream processing, with buffer volumes often required…
Single-use XCell ATF Systems for Continuous Processing: 100% Cell Retention, 80% Faster Set-Up
This webcast features: Christine Gebski, Vice President, Product Management and Field Applications, Repligen In today’s flexible manufacturing environments and to improve manufacturing cost of goods, many companies have implemented single-use equipment. To meet these changing customer requirements, the XCell™ ATF 2 and ATF 6 are now available in single-use formats. The XCell ATF Single-use systems deliver the same cell culture performance as the stainless steel systems but with 8x faster set-up time and a simplified autoclave-free implementation workflow. These elements…
New Genderless Sterile Connection Technology – A Quality By Design (QbD) Approach For Greater Sterility Assurance From Manufacturing To Use
Single-use pre-sterilized systems can potentially offer a higher level of sterility assurance and a lower risk of product contamination by eliminating some of the operational procedures of traditional processing. The importance of this benefit was highlighted by the FDA’s product recalls in 2015 where 78% of recalls were attributed to lack of sterility assurance or to contamination of the drug product, with the primary reason being failure to follow written procedures. A key requirement for multi-component, single-use sterile systems is…
Ask the Expert: Top Five Considerations in Outsourcing to a Biomanufacturing Partner
with Paul Jorjorian In BPI’s 5 October 2016 webcast, Paul Jorjorian (director of global technology transfer at Patheon) discussed his top five considerations for companies outsourcing to a biomanufacturing partner. Jorjorian’s Presentation Selecting a contract and development manufacturing organization (CDMO) is an important decision for many biopharmaceutical companies. Beyond time and cost, here are some other criteria to consider. The number-one issue is quality. Is your company’s definition of quality the same as a CDMOs — and if…
Ask the Expert: Boosting Profits with Single-Use Powder Transfer
with Chris Rombach Open-suite biomanufacturing is gaining popularity. But it leaves surfaces open to contamination, workers at risk for exposure to airborne particulates, and processes more exposed to product loss due to waste or spillage. Liquid-handling systems are often adapted for dry-powder applications, but flow rates, handling characteristics, and dispensing volumes differ dramatically. ILC Dover’s purpose-built, single-use EZ BioPac system is designed specifically to contain and release dry powders. With single-use bags engineered for the task, it can…
Ask the Expert: BioSC Continuous Chromatography of MAbs, Process Design and Regulatory Considerations
with Vincent Monchois In our September webcast, Vincent Monchois (biopharma strategic project director at Novasep) discussed continuous chromatography using BioSC technology. He included regulatory considerations and the challenges of viral clearance that arise when companies switch from batch to continuous processes. Monchois’s Presentation Users can apply BioSC multicolumn, continuous chromatography to standard capture steps such as ion-exchange or protein A chromatography. The main advantages are continuous operation and full access to the total static capacity of a resin.…
Taking Medium and Feed Development Beyond Maximizing Protein Titer to Optimizing Glycan Distribution and Simplifying Process Scale-Up
This webcast features: Serena Fries Smith, Process Science Manager, Thermo Fisher Scientific In the early 2000s when many processes were struggling to achieve 1 g/L, maximizing titers was the industry’s biggest challenge and was essential to having favorable cost of goods and an economically viable product. Over the last 1–15 years, the industry has made significant advances in media and feeds. Due to these advancements, today a standard fed-batch process can typically achieve 3 g/L and some processes are achieving…