Biopharmaceutical companies can develop entire end-to-end single-use production platforms and use them for commercial manufacture of their biological products. Single-use facilities are flexible, can be implemented quickly, and do not require the large up-front capital investments needed for stainless steel equivalents. However, single-use facilities must be supplied with a large quantity of high-quality consumables. Biomanufacturers should pay close attention to their supply chains for those consumables to ensure that they are robust, integral, and fully compatible with biological expression systems…
Sponsored Content
Prescriptive Analytics for Bioprocessing Platforms
The preceding articles have shown how biopharmaceutical companies increasingly are adopting single-use manufacturing technologies for commercial production facilities as their confidence grows in the material science, supply chains, and robustness of single-use systems. Single-use suppliers can support adoption of end-to-end process platforms in commercial manufacturing settings with dedicated teams of experts in process development, engineering, and regulatory support. Advances in process analytical technology (PAT) are providing engineers with greater information on conditions within their single-use bioprocess platforms to allow for…
Final Thoughts: Single-Use Platforms
The development and launch of new biopharmaceutical products is a very challenging and risky process. Sponsors must get their products into clinical testing as quickly as possible to beat their competition and take the greatest share of the market. Yet that need for speed must not lead to companies launching products with inefficient manufacturing processes that ultimately will leave them vulnerable to attack from low-cost competition. Single-use systems have been a significant enabling technology for companies developing new biologics because…
eBook: Advances in Single-Use Platforms for Commercial Manufacturing
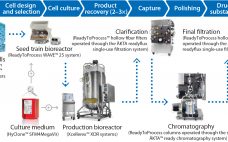
This special report from Sartorius Stedim Biotech explores recent advances in single-use process platforms for commercial production of biopharmaceuticals. Enormous developments have been made in single-use systems, allowing biomanufacturers to adopt the technology in critical applications within their commercial-scale facilities. The authors illustrate how companies can work with teams of experts from supplier organizations to implement end-to-end single-use bioprocesses at scales up to 2,000 L by carefully considering the approach from the earliest stages in process development. Finally, the report…
Are All Protein A Resins the Same? A Performance Comparison of Eight Different Protein A Resins
Protein A affinity chromatography continues to be the preferred method for commercial purification of antibodies due to its very high selectivity and robust resin performance over repeated purification cycles. It is now estimated that over US$125 billion of yearly sales will be generated from monoclonal antibody (MAb) products by 2020 (1). The clear majority will be purified by large-scale protein A affinity chromatography. With the continued growth and therapeutic commercial importance of MAb production, the availability of high-quality resin material…
eBook Download: A New Paradigm in Process Development
As the need to accelerate biopharmaceutical development around the world continues to grow, biomanufacturers face a host of challenges so they, too, can grow. Increasing process productivity, reducing cost, mitigating risk, and bringing products to market faster are just a few of the issues frequently addressed. But with support in process development, cGMP manufacturing and training, accelerating bioprocess development can become less challenging. Several biomanufacturers have successfully navigated these issues in collaboration with GE Healthcare’s Fast Trak Services. Whether…
Planova BioEX: Leading the Next Generation of Virus Filters
This webcast features: Daniel Strauss, PhD, Principal Scientist, Research and Development group at Asahi Kasei Bioprocess America Virus filtration is a critical unit operation in the manufacture of biotherapeutic products owing to its capability to remove a broad range of viruses. Selection of virus removal filters based on filterability and virus removal performance over a range of process conditions ensures predictable processes. The Planova™ BioEX filter shows high flux with minimal flux decay for some of the most challenging products…
Digitization of Biology to Revolutionize Lab Productivity
Biological research is limited by a technical barrier that exists between scientists and lab hardware. Each piece of hardware has its own software, its own file format and its own eccentricities, which the user needs intimate familiarity with to get the most from the machine. Using Antha, a user-friendly but hugely powerful software platform, gives biologists sophisticated, flexible and integrated control over lab hardware. This enables experiments and lab workflows to be rapidly automated, with data automatically integrated and linked…
Introduction: Fast Trak Services Accelerate Global Biopharmaceutical Development
As the need to accelerate biopharmaceutical development around the world continues to grow, biomanufacturers face a host of challenges so they, too, can grow. Increasing process productivity, reducing cost, mitigating risk, and bringing products to market faster are just a few of the issues frequently addressed. But with support in process development, cGMP manufacturing and training, accelerating bioprocess development can become less challenging. Several biomanufacturers have successfully navigated these issues in collaboration with GE Healthcare’s Fast Trak Services. Whether it…
Flavivirus Vaccine Production Accelerates with Modern Bioprocess Tools and Solutions
As with all viral vaccines, the complex nature of flaviviruses makes process development technically challenging. In addition, vaccine production can be both costly and difficult to scale to meet market demands. In egg-based vaccine production, for example, 100–300 vaccine doses can be produced from one fertilized hen’s egg. However, the eggs used for production need to be supplied from special pathogen-free chicken flocks, limiting availability of eggs and making vaccine production difficult to scale up. To meet the needs of…