Whether viral vectors are clarified or the bioburden after cell harvest needs to be reduced to recover antibodies, such applications in biopharmaceutical production require large filtration areas. Single-use technologies are indispensable in many such bioprocesses. Although some single-use filter assemblies have reached their limits, Sartorius Stedim Biotech has made developments to revolutionize these production steps. Scale-Up Limitations in Single-Use technology Conventional stainless steel process systems have been established for decades in the pharmaceutical industry. They are the basis of safe…
Sponsored Content
Establishing Effective High-Throughput Contaminant Removal with Membrane Chromatography
Bharat Serums and Vaccines Limited (BSV) in India conducted a study based on effective removal of host cell proteins (HCPs) from a recombinant hormone with a wide isoform profile in the acidic range imparting drug-product activity. Because the hormone and HCPs have a similar range of active species, the purification process with conventional chromatography resins had difficultly removing those HCPs from the active isoforms of the hormone. To solve that issue, a membrane chromatography technique was implemented. Our initial choice…
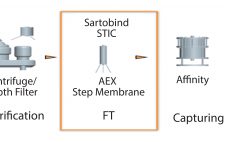
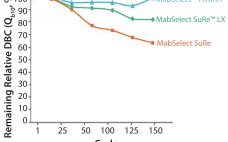
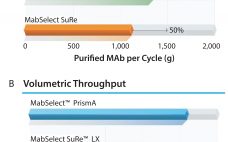
Addressing the Risk of Bioburden and the Need for Increased Productivity in Protein A Chromatography
Protein A has been a fantastic tool for the antibody industry. It is without doubt the most well-established purification technique in monoclonal antibody (MAb) manufacturing today due to a few key success factors. Protein A is the result of the long evolution of Staphylococcus aureus that developed a defense system against antibodies. Protein A exists on the cell wall of about 9% of S. aureus strains and immobilizes IgGs. When there is an immune response in the body, the bacterium…
Evaluation of a Next-Generation Protein A Chromatography Resin for the Purification of Monoclonal Antibodies
Next-generation, high-capacity, alkali-stable protein A resins have recently become available in anticipation of increased production and throughput demand for protein therapeutic processes. Efficient caustic cleaning and bioburden control regimens have allowed agarose-based, alkali-stable protein A chromatography resins to become the backbone of many commercial purification processes for over a decade and have served as the primary capture step for monoclonal antibody (MAb) purification processes. However, the relatively high raw material costs, along with limitations to binding capacity and aggressive resin…
Reimagining Capacity for Today’s Purification of Monoclonal Antibodies
The monoclonal antibody (MAb) market has grown over the past decade to be about half of the biomanufacturing market today. This growth should continue, driven by a strong pipeline of MAbs that are currently in phase 2 and 3 clinical trials. A synergistic evolution of MAbs and protein A resins also has taken place in the market. Figure 1 shows a graph adapted from an Amgen study of the development of protein A productivity and capacity over the past 40…
Thirty Years of Monoclonal Antibodies and Protein A: A Retrospective
In 1980 at the University of Cambridge’s department of pathology, I worked with Herman Waldmann to develop monoclonal antibodies (MAbs) as treatments for graft-versus-host disease (GVHD). That disease is associated with serious complications of stem cell transplantation when attacking T-cells can damage the lungs, liver, skin, and other organs. If we could find a specific MAb that would work with the human complement system to kill those cells, they could be selectively removed from the bone marrow. The human complement…
A Discussion Regarding the Productivity in Downstream Operations
At the Biotech Week Boston conference (24–28 September 2017), BioProcess International publisher Brian Caine had the opportunity to speak with Jonathan Royce, global business leader for chromatography resins at GE Healthcare. Their discussion covered current downstream challenges and the company’s next-generation chromatography resin. Industry Challenges Caine: What are the most critical purification challenges for biomanufacturers? Royce: The bioindustry has seen more than a 100-fold increase in the productivity of cells. This means that downstream purification technology must continue to evolve…
High-Throughput Technologies: Accelerating Process Development
One of the key elements of any biopharmaceutical drug development project is the timeline from identification of the appropriate DNA sequence to investigational new drug (IND) application filing and the start of clinical trials. Typically, this timeline ranges from 18 to 20 months, depending on the type of molecule being developed and the extent and requirements of the chemistry, manufacturing, and controls (CMC) packages supporting the nonclinical and clinical parts of a development program. There is constant pressure to shorten…
We Meet Your Single Use Process Monitoring Requirements
Innovation is at the core of all pharmaceutical, biopharm and bioprocess applications. With extensive experience in the biopharmaceutical industry — and particular expertise in single-use bio-pharmaceutical equipment — PendoTECH delivers a line of pressure sensors, control systems and software for measuring, monitoring and data collection in bioprocess applications and other areas where the products provide a unique process solution. Committed to meeting the specialized needs of life-science laboratories and bio-pharmaceutical manufacturers, PendoTECH technical consultants review your company’s applications in the…
U.S. Approval of Three Rapid Microbiological Methods for MACI Product Release
Short time frames are a major challenge in developing alternative microbiological methods for autologous cell therapy products. Ideally, results are made available in under a day. Obtaining regulatory acceptance also can be a challenge, but it is made easier if methods are included in an application (e.g., a biologics license application, BLA) rather than changing a method that is already part of an approved process. Comparing different detection platforms can be a challenge if they have different readouts, and validation…