Turn-key single-use aseptic sampling devices (ASDs) have diminished bioprocess contamination risks significantly. But depending on testing, facility, and storage needs, some ASD container types are more effective than others are. Bobbi Allen (technology expert at Sartorius Stedim Biotech North America, SSB) focused her 8 January 2020 “Ask the Expert” presentation on “what, why, when, and where” operators must sample aseptically from stainless-steel tanks. Using data from in-house testing of aseptic sampling containers, Allen offered key considerations for sterility, process monitoring,…
Sponsored Content
Improving Bioprocess Expression Systems: A Clean Alternative to CRISPR/Cas9
Chinese hamster ovary (CHO) cells have emerged as a robust platform for bioprocessing serving both early and late-stage biotherapeutic drug supply. However, these cells and other hosts (e.g., HEK293), can be optimized for even greater potential through advanced gene editing. For example, when the endogenous glutamine synthetase (GS) gene is knocked out in CHO cells, a sixfold increase in high-producing cell lines is achieved (1). In another study, CHO with annexin A2 (ANXA2) and cathepsin gene (CTSD) knockouts were introduced…
Introducing New Digital Tools to Enhance Raw Material Verification
This webcast features: Antonia Guerra, Global Digital and Data Science Leader, Field & Safety Instruments, Thermo Fisher Scientific™ This webinar presents the new virtual companion to the Thermo Scientific™ TruScan™ RM Handheld Raman Analyzer, the Virtual TruScan™ RM (VTR) App, which creates efficiencies in material identity verification. This first-of-its-kind digital tool puts the TruScan RM’s decision algorithm in the cloud and allows for method validation and spectral re-processing without the need for a physical sample. With the VTR app, wait…
Bioprocess Development and Qualification: PAT-Based Stage 1 and 2 Acceleration Strategies
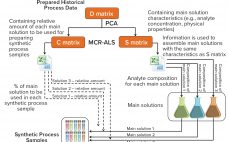
Well-established process analytical technology (PAT) strategies, such as those based on spectroscopy, bring with them several challenges related to the nature of those tools themselves (1–3). Such tools are multiparametric by design — in the sense that most spectroscopies capture multiple attributes sometimes different in nature (e.g., near-infrared, NIR, captures chemical and physical attributes simultaneously). Often a reference method is required; at other times, indirect calibrations are based on the correlation of one culture attribute with another for which a…
Ask the Expert: Highly Sensitive Host-Cell Protein Analyses Using Novel Chromatography Technology
Geert Van Raemdonck (global field support expert at PharmaFluidics) and Koen Sandra (scientific director of the Research Institution for Chromatography, RIC) teamed up for a 10 October 2019 “Ask the Expert” webinar to introduce micro Pillar Array Column (ÎĽPAC™) technology for liquid chromatography–mass spectrometry (LC–MS) for host-cell protein (HCP) detection. Van Raemdonck explained that ÎĽPAC technology approaches chromatography differently than does packed-bed technology. Microfluidic channels with arrays of free-standing pillars are etched lithographically into a silicon wafer. The resulting permeability…
Ask the Expert: Accelerating Timelines By Integrating Cell-Line Development and Manufacturing
In a 31 October 2019 “Ask the Expert” presentation, Nicole Wakes (group leader of Abzena’s cell-line development team) observed that drug sponsors often outsource their early upstream activities to a few different contract research organizations (CROs). But that strategy can thwart short timelines and introduce regulatory and financial risks. Wakes described Abzena’s upstream approach, illustrating how partnering with a single, multicompetent CRO from cell line construction through manufacture can streamline workflows. Integrating cell line development and manufacturing in this way…
Ask the Expert: Developing Bioprocesses for Clinical Manufacturing Success
Biopharmaceutical companies need to make critical chemistry, manufacturing, and controls (CMC) decisions during clinical development of recombinant protein biologics and advanced therapies. In a 17 December 2019 “Ask the Expert” webinar, Nigel Shipston (director of program design at FUJIFILM Diosynth Biotechnologies, FDB) reviewed key aspects of selecting and working with a contract development and manufacturing organization (CDMO). He also highlighted important factors that should be considered during early stages of process development. Shipston’s Presentation The sheer magnitude of investment required…
Ask the Expert: Cell Culture Media Analysis Using Handheld Raman Analyzers
In biopharmaceutical manufacturing, cell culture media supply critical nutrients and maintain pH and osmolality to optimize protein product yield. Because media composition and condition have a strong effect on final biologic product quality and production, biopharmaceutical companies monitor media for lot-to-lot variability. Stability testing for degradation due to light exposure, temperature changes, or shelf-life/time is possible with rapid spectroscopic methods. In an 8 October 2019 “Ask the Expert” webinar, O. Dean Stuart (product manager at Thermo Fisher Scientific) explained how…
Automated Cell Line Development With Greater Than 99% Monoclonality On the Beacon® Platform
For all cell lines used in production for commercial therapeutics, FDA guidelines require assurance of monoclonality. This standard means that typical cell line development (CLD) campaigns often require multiple rounds of cloning easily spanning several weeks to months. However, Berkeley Lights, Inc. (BLI) has developed the ability to save time and money spent on CLD processes via automation. The uniquely powerful Beacon® optofluidic platform performs single-cell cloning, growth and titer measurements, and recovery of top clones with unrivaled (>99%) monoclonality…
Upstream: Make the Right Decisions for Your mAb
Bioprocess decisions made during upstream operations can be difficult to reverse at later, more costly stages of biologic manufacture. They even can require significant backtracking, wasting precious time, labor, and material. Read this Special Report to learn ways to optimize monoclonal antibody bioprocessing upstream. Specifically, you will learn about different tools that small and emerging biotechnology groups can use to ensure robust cell-line selection novel media formulations designed for intensified upstream processing in perfusion modes mixing and delivery solutions that…