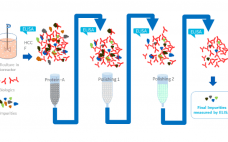
Host cell proteins (HCPs) are a primary source of impurity in biologics manufacturing. When present in drug formulations, HCPs can reduce efficacy, introduce toxicity, and increase risk of long-term immunogenicity. Understanding HCP profiles and integrating effective removal strategies are critical when developing a new biological drug, both for ensuring patient safety and fulfilling regulatory guidelines. HCP populations can be complex and structurally diverse, and most changes in upstream culture conditions affect HCP concentrations and control strategies. Accurate and reliable HCP…
Sponsored Content
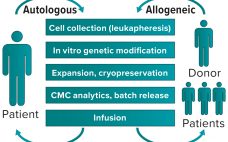
Transforming Personalized Medicine into Off-the-Shelf Cell Therapies
Initial progress in cell and gene therapy has seen 12 advanced therapeutic medicinal products (ATMPs) become available on the market in 2019 for a range of conditions, from monogenic diseases to cancer. Despite such progress, development of clinically and commercially successful cell therapies presents manufacturability challenges and questions about bypassing patients’ immune systems. The availability of rapid sequencing and next-generation bioinformatics has made it possible to understand the mechanisms of disease better and accelerate development of therapeutic responses. The same…
A Rapid, Low-Risk Approach Process Transfer of Biologics from Development to Manufacturing Scale
Successful scale-up of cell culture for manufacturing of biopharmaceuticals gives companies time to accelerate clinical development, product commercialization, and market access (1). Scaling a cell culture process in stirred-tank bioreactors ideally includes optimizing that process at laboratory scale and then transferring it through larger pilot-scale and finally to manufacturing-scale bioreactors (2). This is a complex, time-consuming business that can involve process transfer — sometimes to different geographical locations and through many sizes of bioreactors, each of which can operate according…
Sharing Viral Vector Expertise: A Conversation with Yposkesi’s Chief Executive Officer
As a full-service contract development and manufacturing organization (CDMO) specializing in gene therapy development, Yposkesi produces recombinant adenoassociated virus (AAV) and lentivirus (LV) vectors using adherent-based and suspension-adapted cell expression platforms. Alain Lamproye joined the company as chief executive officer (CEO) in January 2017, having served previously as president of the biopharmaceutical business unit of Novasep (2012–2017) and as CEO of Henogen, its subsidiary dedicated to gene therapy. He has held managerial positions in pharmaceutical operations at Merck Serono (including…
Ask the Expert: Key Considerations for Cryogenic Preservation and T-Cell Viability
Cryopreservation provides critical protection for cell therapies by minimizing genetic changes. But cooling too slowly or quickly risks diminishing cell viability upon thaw. On 11 March 2020, Peter Kilbride (senior research scientist) and Julie Meneghel (cryobiologist), both of Cytiva (formerly GE Healthcare Life Sciences), discussed the importance of controlled-rate cryopreservation. Illustrating how mammalian cells change when frozen, Kilbride and Meneghel offered concrete cryopreservation strategies and identified temperatures at which it is safe to stop controlled cooling and transfer drug product…
Ask the Expert: A Robust, Stable Platform for Biologics Development
Sean Liour (vice president for project management at GenScript ProBio) delivered an Ask the Expert presentation on 18 March 2020 to explore the advantages of his company’s platform for cell-line development. Liour explained that successful biologic development hinges on robust host cell lines, capable expression vectors, and discriminating clone-screening systems. Overviewing relevant technologies and capabilities, Liour illustrated how his company’s ProCLD platform helps sponsors navigate cell-line development. Liour’s Presentation Researchers must weigh their options carefully when selecting a host cell…
Cyclic Cell Harvest with CONTIBAC® SU Filters
As the Biotech industry is moving towards single-use components, larger batch volumes and higher cell concentrations, conventional cell harvest technologies reach their limit. Depth filters, for instance, can only cope with the increasing demands by stacking more filter elements and therefore increasing the footprint and their economic burden. Hence, innovative solutions are needed to keep pushing the boundaries of the biologics production. The CONTIBAC® SU filter of DrM excels where existing technologies crumble. Unlike in any competing technology, the filtration…
Navigating Technology Transfer
Technology transfer is a key milestone in the journey from discovery to full-scale good manufacturing practice (GMP)-compliant manufacturing. Navigating this step while preventing unforeseen issues that can create costly delays is supported best by combining knowledge of a given process with understanding of the technological capabilities. Different applications have different needs. Some challenges and goals are common to bioreactor processes for suspension and adherent cell culture for production of viral vectors, monoclonal antibodies (MAbs), other recombinant proteins, and vaccines. All…
Innovative Closed Process CAR-T Cell Therapy Platform to Streamline Approach for Manufacturing with Great Predictability
This webcast features: Tatiana Golovina, Senior Director, Cell Therapy Process Development, WuXi Advanced Technologies For many years, the primary forms of cancer treatment have been chemotherapy, radiation, and surgery. An amazing breakthrough known as chimeric antigen receptor (CAR) T-cell therapy is being studied in the treatment of various types of cancer, including acute and chronic lymphoblastic leukemia, non-Hodgkin lymphoma, myeloma, and solid tumors. Developing innovative advanced therapies is one of our greatest opportunities to dramatically improve patients’ lives. WuXi Advanced…
Assessing Viral Clearance in Early Phase Process Development
This webcast features: William H. Rushton, Process Chromatography Support Scientist, Bio-Rad Laboratories Viral clearance studies are part of a multifaceted approach to ensure the safety of biopharmaceutical products. In order to prevent costly changes to a manufacturing process, it is important to assess each operation unit for its efficiency on the removal or inactivation of adventitious agents early on during downstream process development. A design of experiments (DOE) approach was utilized in this case study to investigate the effect of…