Thomas Gabriel (director of strategy and business development at Fujifilm Diosynth Biotechnologies, FDB) emphasized patient agency in his 15 April 2020 “Ask the Expert” presentation on innovations in finished-goods solutions. Facilitating self-administration significantly benefits patients with chronic conditions, and new delivery technologies are making drug products increasingly easy to handle and with increasingly accurate dosing. Gabriel explored how single-use autoinjectors and prefilled syringes, needle shields, on-body delivery systems, and digital monitors are improving drug-delivery safety and efficacy while enabling patients…
Sponsored Content
Ask the Expert: Improving Host-Cell Protein Detection By Enriching Mass Spectrometry Samples
Enzyme-linked immunosorbent assays (ELISAs) remain the industry standard for process monitoring and lot-release testing for host-cell proteins (HCPs), but researchers need orthogonal tests to confirm that an ELISA is fit for purpose. On 22 April 2020, Eric Bishop (vice president of research and development at Cygnus Technologies) delivered an “Ask the Expert” presentation about his company’s solution: mass spectrometry (MS) preceded by a novel Antibody Affinity Extraction (AAE) technique. Bishop explained that an AAE step can magnify a sample’s HCP…
Managing Risk in Single-Use Systems Design and Implementation: A Shared Responsibility
Managing risk in single-use systems design and implementation is a shared responsibility. The ultimate responsibility for drug processes and products will always remain with manufacturers. However, implementation of single-use systems can shift responsibilities to suppliers within key areas, including design and sterilization, which must be clearly controlled and validated. This Special Report discusses how suppliers and manufacturers when working together can mitigate the risk of applying single-use systems in biopharmaceutical production from design through validation to point-of-use testing and operator…
2.5Ă— Lentiviral Vector Bioreactor Yield Increase and Simplified Execution with Innovative Tangential-Flow Depth Filtration
This webcast features: Michael Bransby, R&D Director of Process Technology, Repligen In a joint collaboration, Oxford Biomedica and Repligen increased the yield of viral vectors from suspension-cultured bioreactors several fold using tangential-flow depth filtration (TFDF). The yield for a single clarification step was 95% as compared to 70% by standard depth filtration. The TFDF tubular format and low shear enabled further yield increases through multiple harvests from the same seeding. A single seeded bioreactor produced two harvests of 95% and…
Confidence in Host Cell Protein (HCP) Coverage Assays with Differential Gel Approaches
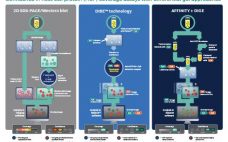
Enzyme-linked immunosorbent assays (ELISAs) are critical to detecting and removing host cell proteins (HCPs), a primary source of impurities in biologics development. Scientists must validate HCP ELISAs to ensure patient safety and meet regulatory guidelines – but different coverage assays come with different challenges, limitations, and benefits. The US Pharmacopeia recommends 2D differential gel electrophoresis (DIGE) combined with Western blot or immunoaffinity approaches, and labs might not be able to gain the full benefits of DIGE without significantly altering current…
Cytiva shares tips for viral vector production
The viral vector market is highly active, and the interest in production technologies is driven by recent approvals in cell and gene therapy, says Ă…sa Hagner-McWhirter, senior scientist at Cytiva. Demand for viral vector production is high. The main driver is the large pipeline in different clinical stages and scales. In 2018 there were 724 clinical trials for gene therapies and gene-modified cell therapies, many of which use viral vectors. Adenoviruses and retroviruses, including lentivirus, are frequently used. Adeno-associated virus…
Optimizing Your Excipient Screening for Vaccine Formulation with an Ultra-Pure Pharmaceutical Gelatin
This webcast features: Jeroen Geeraerts, Business Development Manager, Biomedical, Rousselot The world is working at an unprecedented pace to develop a safe and effective vaccine to combat the COVID-19 pandemic. Currently, five candidate vaccines are in clinical evaluation, and many more are in preclinical testing. Different types of vaccines are being developed using multiple strategies and platforms. Among them are several inactivated-virus and live-attenuated–virus candidate vaccines. As an excipient, gelatin is a key component in many vaccine formulations. Well-known examples…
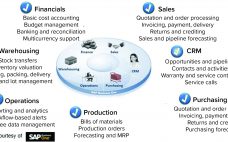
Challenges and Benefits of Networking Process Control Manufacturing Systems: Integration to Business Systems in Industry 4.0
Networking of manufacturing process control systems can lead to benefits of efficiency, increased productivity, and better facility use, leading to lower cost of goods (CoG). Furthermore, integration of manufacturing systems with manufacturing execution systems (MESs) upward to an enterprise resource planning (ERP) business system improves overall organizational efficiency. An ERP system enables optimization of intrafacility manufacturing resources. For multiple manufacturing facilities, it facilitates optimization across a company’s manufacturing network. Enabling Technologies and Systems At the enterprise resource planning level (Figure…
Protein A Chromatography: Using Automation Instead of Multiple Columns To Increase Productivity
This webcast features: Van Leang, Senior Director of Global CMC Operations, HJB Bio Vendors supporting biologics manufacturing are developing equipment to enable continuous process in downstream operations. Using continuous downstream operations reduces operational time and raw material costs. HJB has developed a downstream hybrid process that uses an automated batch approach to achieve a continuous downstream capture process. This allows the use of the current monoclonal antibody (MAb) capture platform but reduces cost overall by adopting the benefits of continuous…
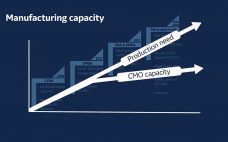
Technology-Ready Processes for Gene Therapy Manufacturing 2.0
This webcast features: RenĂ© Gantier, PhD, Director of Technology, Gene Therapy, Repligen The current manufacturing processes for viral vectors for gene therapy, which we can define as Gene Therapy Manufacturing 1.0 (e.g., adherent cell culture and transient expression from plasmid transfection), are not productive enough to meet the future demand considering the quickly increasing number of approved gene therapies and clinical trials. A transition is therefore ongoing to implement more productive and scalable processes, leading to Gene Therapy Manufacturing 2.0 using…