On 1 December 2020, BPI presented an “Ask the Expert” webinar with Tanner Nevill (vice president of program management at Berkeley Lights). Monoclonality assurance is a central regulatory requirement for all cell lines producing biologic therapies. Well-plate imaging is the “gold standard” for confirming that a cell line originates from a single cell, but it is labor intensive and prone to errors in the form of “ghost cells” and debris that look like cells. Using the Berkeley Lights Beacon system, cells…
Sponsored Content
FAQs: Challenging host cell protein assays for improved risk mitigation
Host cell proteins (HCP) are critical to quality control in biologics development. If left unremoved, they can cause immunogenic responses and reduce drug efficacy. In our last webinar we shared Cytiva’s strategy for designing a comprehensive HCP risk mitigation strategy. We discussed how to challenge your assays early to ensure accurate measurements, build confidence, and reduce the risk of unexpected HCP levels. Our HCP experts highlighted and responded to the most common questions raised around host cell protein assays. Want…
The next frontier of CAR-T cell therapies: Allogeneic workflows
The development of CAR-T cell therapies provides treatment options for some of the most vulnerable cancer patients, giving them a fighting chance to battle their diagnoses if they’re eligible. Researchers and organizations continue to work diligently to optimize the manufacturing process to develop safe and effective CAR-T cell therapies as efficiently as possible, with the hope that one day they can be manufactured at a lower cost and made available to any patient who could benefit from them. Personalized therapies…
Reducing Risk in Bioproduction with Facilities Equivalency
Providing consistency in cell culture media biomanufacturing is critical to supply continuity. Central to this is the development of redundancy and harmonization across a global manufacturing network. These unprecedented times have also highlighted the importance of strategizing for increased and unexpected demand. Read this Special Report to learn about the importance of equivalency and the strategies used to maintain this critical requirement at Gibco cell culture media manufacturing facilities. Fill out the form below to read the complete report and…
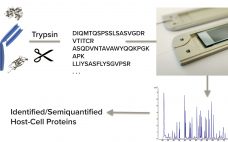
Nontargeted HCP Monitoring in Downstream Process Samples: Combining Micro Pillar Array Columns with Mass Spectrometry
Protein biopharmaceuticals have emerged as important treatments for diseases with otherwise unmet medical needs. These biologics are produced by recombinant mammalian, yeast, or bacterial expression systems. Along with therapeutic proteins, those cells produce endogenous host-cell proteins (HCPs) that can contaminate biopharmaceutical products despite multiple purification steps in downstream processing. Because such process-related impurities can affect product safety and efficacy, they need to be monitored closely. Multicomponent enzyme-lined immunosorbent assays (ELISAs) presently are the workhorse method for HCP testing, with high…
Fragment-Based Screening in Drug Discovery: How to Improve Hit Rates & Deliver Higher-Value Targets
Learn about the basics of fragment-based drug discovery (FBDD), one of the most valuable approaches to small molecule screening and now even more widely used today by pharma companies than high-throughput screening (HTS) HTS’s main limitations — low success rates for more challenging targets, high level of false positives, and the size of the compound libraries — are propelling the use of FBDD. This change is also driven by the rise in the number of explorative targets, which demands not…
The Watershed Moment for ADCs Has Arrived — Now, CDMO Selection Is the Last Barrier
As far back as 10 years ago, antibody–drug conjugates (ADCs) were being talked about as the next big breakthrough in pharmaceuticals due to their highly targeted approach to therapies, especially in areas such as oncology. When Adcetris (brentuximab vedotin) became the second ADC approved by the US Food and Drug Administration (FDA) in 2011, it was predicted to be a watershed moment when the floodgates would open for more approvals and novel ADC approaches. Despite that possibility, the approval of…
Analytical Support for Biologics: A Conversation with Almac Sciences
Almac Sciences (a member of the Almac Group) recently expanded its suite of analytical solutions to include biologics testing. This follows a 2019 expansion of the company’s facility in Athlone, Ireland, where it provides a comprehensive range of flexible pharmaceutical testing services to support drug development programs adhering to industry regulations and good manufacturing practice (GMP) standards. “Biologics have gained huge traction in the past decade and are poised for stronger growth in the coming years with potential to significantly…
Ultrasonic Flow and Bubble Sensors: Optimize Process Quality in Single-Use Bioprocessing Applications
Process monitoring in laboratory and production environments enables continuous control and optimization of critical process parameters. Hence, the early detection of errors is an effective means of increasing process efficiency and reproducibility, improving quality and safety parameters, and reducing long-term costs. Highly precise and flexible noncontact clamp-on flow and bubble sensors are useful instruments to fulfill these goals. They can be applied effectively to buffer and media preparation, chromatography and filtration systems, bioreactors and fermentors, feed and transfer lines, and…
Evolving Business Models to Meet the Future of Healthcare
Yourway provides integrated supply-chain solutions for the global pharmaceutical and biotechnology industries. We exceed traditional offerings to provide customized support that includes warehousing and packaging, project management, planning and optimization guidance, comparator sourcing, ancillary-supply sourcing, forecasting, and returns/reconciliation management. Our single–specialized-provider approach offers clients a high level of convenience and efficiency that translates into quality, speed, and operational improvements. A Growing Global Footprint As we introduce new added-value solutions, expand our global footprint, and continue investing in infrastructure, technology, training,…