The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guideline Q12 (1) (step 4 sign-off in November 2019) is in the process of being implemented in a number of regulatory regions. The document provides additional frameworks for pharmaceutical life-cycle management. It is intended to support globally harmonized regulatory tools such as established conditions (ECs) and product life cycle management (PLCM) documents to facilitate postapproval changes to chemistry, manufacturing, and controls (CMC). Although a harmonized framework…
Manufacturing
Designing Vaccines: The Role of Artificial Intelligence and Digital Health, Part 1
According to the founder and executive chairman of the World Economic Forum, Klaus Schwab, the fourth industrial revolution began in the 21st century and is characterized by an unprecedented development and exponential growth of a high-technology industry transforming society at every level (1–4). In particular, healthcare systems are evolving rapidly to adapt to the new reality. According to Forbes, the main technologies currently shifting the paradigm of medical research are artificial intelligence (AI) and machine learning (ML) (5), both defined…
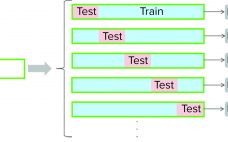
Multivariate Data-Driven Modeling for Continued Process Verification
Continued process verification (CPV) is an integral part of process validation for the manufacture of human and animal drugs and biological products (1). It is designed to meet three primary goals: maintain a validated state of products, their processes, and related systems; enable continuous process improvements; and meet regulatory requirements for life-cycle validation. A CPV program for a biologic product entails regular collection of data related to critical process parameters (CPPs) and critical quality attributes (CQAs) and the preprocessing, analysis,…
Transfection Best Practices for AAV Gene Therapy Programs
As viral vectors continue to push gene therapy innovations closer to market, many researchers are setting their sights on optimizing transfection, the process of delivering corrective genetic material into cells. It’s not just a question of how to transfect them, but also how to do so efficiently and at high volumes. Approaches that work for one cell line might not perform well for others, and transfection protocols can have different implications for scalability and cost during production for clinical trials.…
Focusing on the Patient Journey Can Increase Access to Lifesaving Therapies
Cell and gene therapies (CGTs) are positioned currently as last-chance, “miracle” cures for patients who have severe illnesses. Such promises require innovation. Despite the cutting-edge science and significant investment that goes into CGT development, fundamental challenges remain, including patient access. The highly personalized nature of autologous-therapy development presents myriad logistical, financial, and manufacturing challenges to ensuring global access to treatment. Understanding a patient’s journey to treatment is vitally important to achieving that goal. Barriers to Cell and Gene Therapy Access…
Recombinant Proteins for Cell and Gene Therapy Research: A Conversation with Shenandoah Biotechnology
Recombinant proteins such as growth factors and cytokines are essential for cell therapy, gene therapy and regenerative medicine research, development and manufacturing. These proteins are critical in the production of desired cell types and subsequent differentiation of cells, to deliver the desired effect. Founded 15 years ago, Shenandoah Biotechnology applies a proprietary method of folding and purifying recombinant proteins from both bacterial and mammalian systems to enable cost-effective, large-scale production of Cell Therapy Grade proteins to support these groundbreaking treatments.…
eBook: Rare Diseases — Biopharmaceutical Challenges Presented By Relatively Small Patient Populations
By definition, an orphan disease affects a small percentage of the population. However, with some 7,000 such conditions identified so far, they collectively have a significant impact on global health. An estimated 350 million people are affected worldwide by a rare disease — altogether more than the population of the world’s third largest country (the United States). Some well-known biopharmaceutical companies are devoted to developing treatments for rare diseases. However, the vast majority of such diseases have no treatments approved by…
Aseptic Considerations in Formulation, Fill and Finish: Choosing Between Barrier and Isolator Technologies
Biological drug substances are constituent in a wide range of medicinal products with an even broader spectrum of applications. Those include autoimmune-disease treatments (e.g., for arthritis), vaccines, and recombinant therapeutic proteins (e.g., for cancer treatment). What such products all have in common is that they are manufactured using biotechnology and other cutting-edge technologies. Biologics are not as physically robust as their small-molecule counterparts. Hence, during biomanufacturing processes, these complex molecules present a number of challenges. Some of the typical shared…
Creative Formulation: A Useful Approach to Patient-Centered Drug Development
The development of new medicines is a highly regulated process focused on demonstrating efficacy and safety of new products. Although such qualities always will remain the primary focus of drug development, the biopharmaceutical industry gradually has adopted additional design aspects. New approaches can help meet patients’ divergent needs to improve their lives in meaningful ways. Often referred to as “patient-centered” or “patient-focused” drug-product design, such considerations are expected by many experts to become an increasingly dominant part of future drug…
Analysis of Trace-Level, High-Risk HCPs: Proteomics Advances for Preventing Degradation of Polysorbates in Biotherapeutic Formulations
Polysorbate-80 (PS-80) and polysorbate-20 (PS-20) are used widely in formulation of biotherapeutic products for preventing surface adsorption and as stabilizers against protein aggregation (1). Degradation of polysorbates can cause turbidity and potential formation of subvisible particles mainly consisting of poorly soluble hydrophobic free fatty acids (1). Polysorbate degradation is an industry-wide challenge both in biotherapeutics processing and formulation development. The risk of such degradation increases with higher cell densities and greater expression titers in bioprocessing, as well as with higher…